
Select the correct statement:
(a) ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ is pyramidal while ${{C}^{-}}{{(CN)}_{3}}$ is planar.
(b) ${{C}^{-}}{{(CN)}_{3}}$is pyramidal while ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ is planar.
(c) Both ${{C}^{-}}{{(CN)}_{3}}$and ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ are planar.
(d) Both ${{C}^{-}}{{(CN)}_{3}}$and ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ are pyramidal.
Answer
567.9k+ views
Hint: The negative charge on the carbon indicates the presence of a lone pair of electrons on the carbon atom. If the compound can have resonating structures then the geometry of the compound will change.
Complete answer:
We are given two compounds ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ and ${{C}^{-}}{{(CN)}_{3}}$. In both the compounds there is a negative charge on the carbon atom. The negative charge on the carbon indicates the presence of a lone pair of electrons on the carbon atom.
In ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$, there are four substituents attached to the carbon atom, i.e., three methyl group and one lone pair. Since there are four substituents the geometry is expected to be tetrahedral but the lone pair causes repulsion on the bond pairs which causes a change in the geometry. The shape will become pyramidal. The structure is given below:
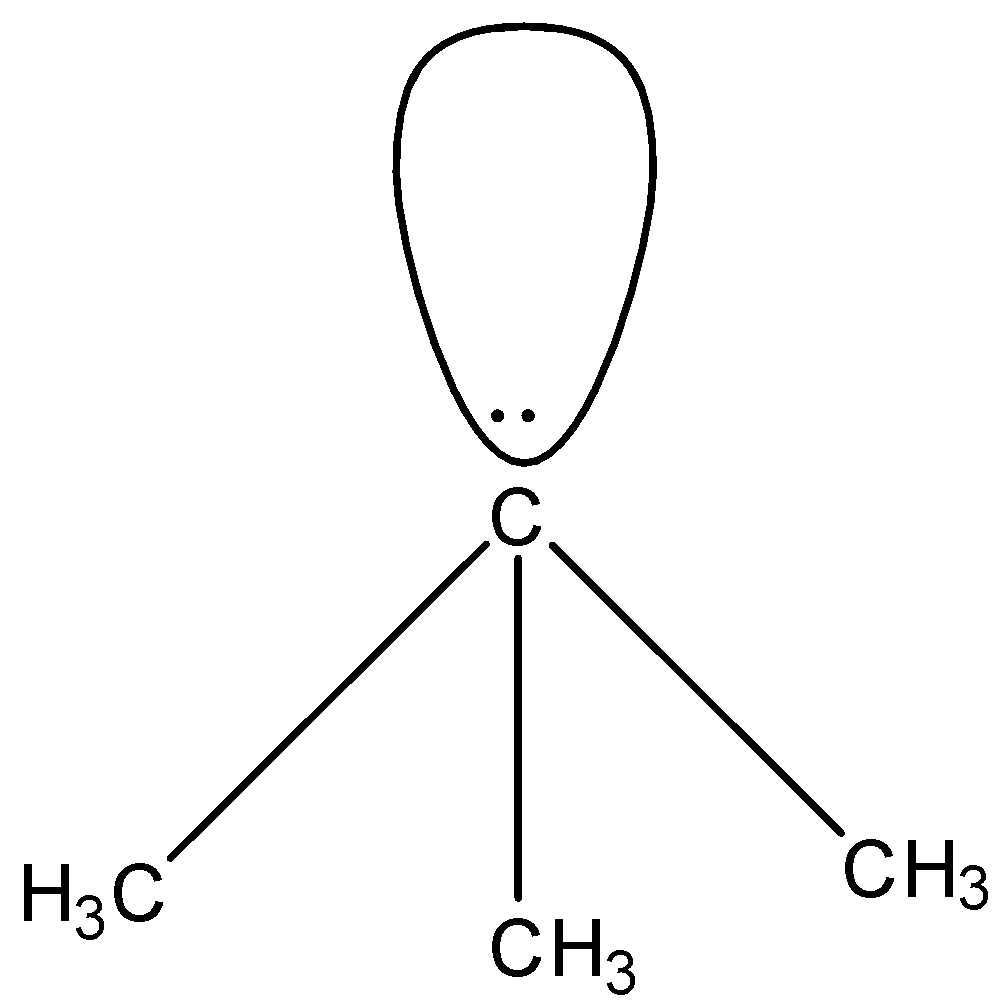
In ${{C}^{-}}{{(CN)}_{3}}$, there are four substituents attached to the carbon atom, i.e., three cyanide group and one lone pair. The cyanide group has a carbon atom and nitrogen atom whose electronegativity is higher than the carbon atom. So, the nitrogen can attract a shared pair of electrons towards itself, hence the lone pair on the carbon atom will start to move on the other atoms. This will cause resonating structures, due to which the shape of this molecule will be trigonal planar. The structure is given below:
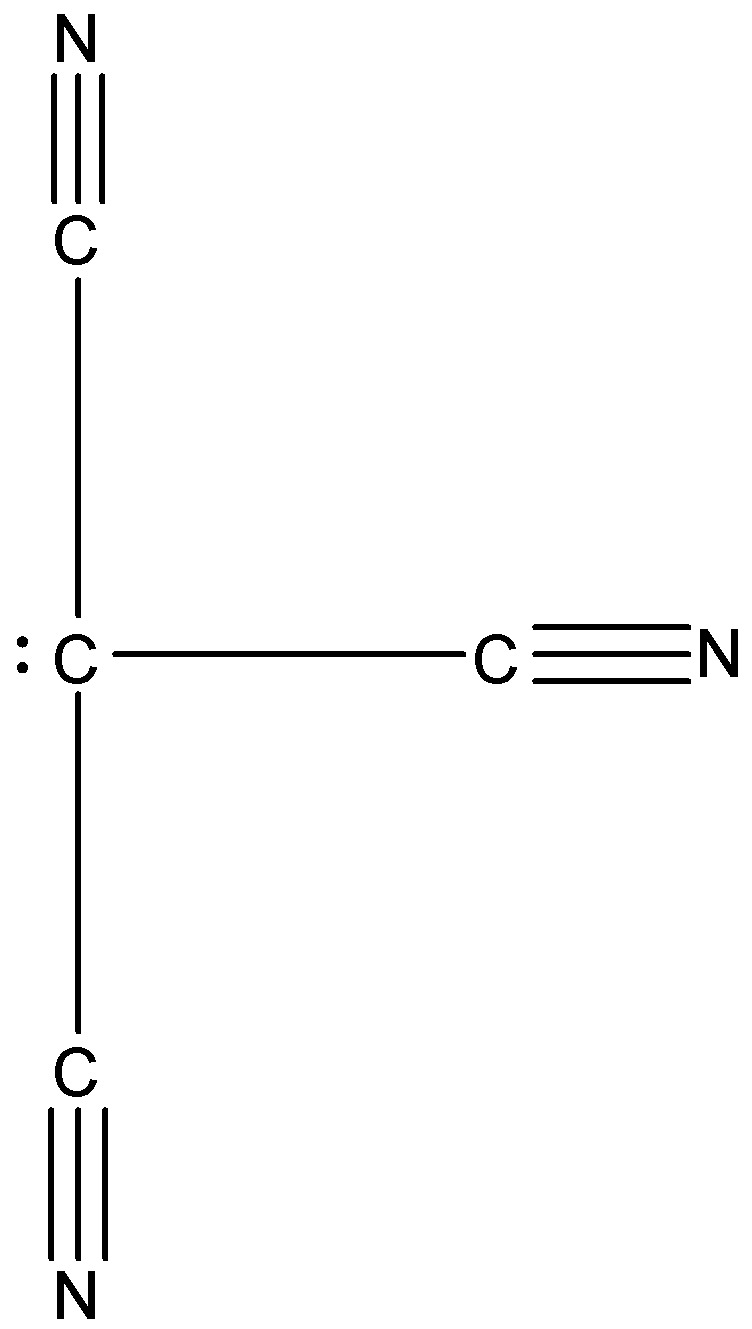
Therefore, the correct answer is an option (a).
Note:
It must be noted that the repulsion between the bond pair-bond pair is less than the repulsion between the lone pair-bond pair. This will decrease the bond angle. The structure of ${{C}^{-}}{{(CN)}_{3}}$ will be a trigonal planar because only three substituents will be counted and for three, the trigonal planar is formed.
Complete answer:
We are given two compounds ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$ and ${{C}^{-}}{{(CN)}_{3}}$. In both the compounds there is a negative charge on the carbon atom. The negative charge on the carbon indicates the presence of a lone pair of electrons on the carbon atom.
In ${{(C{{H}_{3}})}_{3}}{{C}^{-}}$, there are four substituents attached to the carbon atom, i.e., three methyl group and one lone pair. Since there are four substituents the geometry is expected to be tetrahedral but the lone pair causes repulsion on the bond pairs which causes a change in the geometry. The shape will become pyramidal. The structure is given below:

In ${{C}^{-}}{{(CN)}_{3}}$, there are four substituents attached to the carbon atom, i.e., three cyanide group and one lone pair. The cyanide group has a carbon atom and nitrogen atom whose electronegativity is higher than the carbon atom. So, the nitrogen can attract a shared pair of electrons towards itself, hence the lone pair on the carbon atom will start to move on the other atoms. This will cause resonating structures, due to which the shape of this molecule will be trigonal planar. The structure is given below:

Therefore, the correct answer is an option (a).
Note:
It must be noted that the repulsion between the bond pair-bond pair is less than the repulsion between the lone pair-bond pair. This will decrease the bond angle. The structure of ${{C}^{-}}{{(CN)}_{3}}$ will be a trigonal planar because only three substituents will be counted and for three, the trigonal planar is formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




