
Select the acid with the highest ${{K}_{a}}$(i.e. lowest$p{{K}_{a}}$)
A.
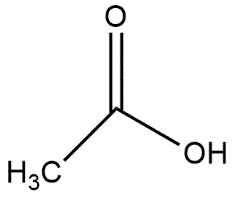
B.
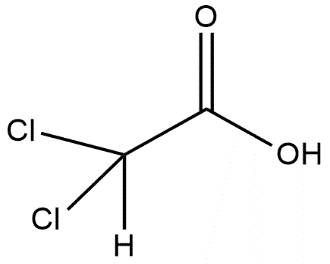
C.
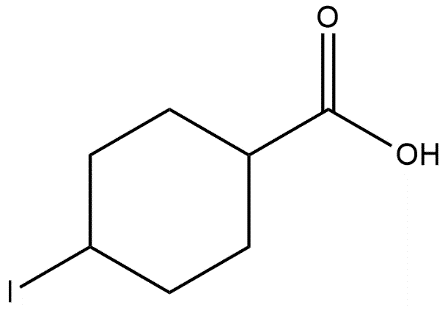
D.
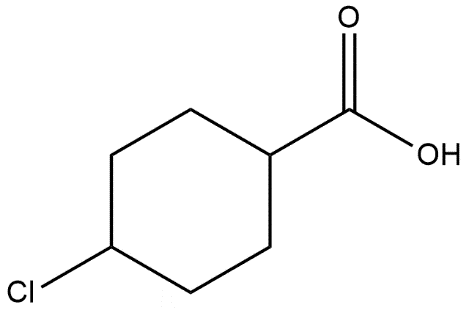




Answer
531.3k+ views
Hint: \[{{K}_{a}},p{{K}_{a}},{{K}_{b}},p{{K}_{b}}\] values are helpful to predict whether a species will donate or accept protons at a specific pH value. These terms describe the degree of ionization of an acid or base. ${{K}_{a}},p{{K}_{a}}$ relate to acids while ${{K}_{b}},p{{K}_{b}}$deal with bases.
Complete answer:
When any electron releasing group or electron withdrawing group is attached with a chain of carbon atoms then partially positive or negative charge arises on the carbon chain atoms which causes permanent dipole in the molecule this type of effect is known as inductive effect. There are two types of inductive effect known as +I effect and –I effect.
-I effect occurs when an electronegative atom like halogen is attached to a chain of atoms resulting in unequal sharing of electrons which generates a positive charge. It causes a permanent dipole in the molecule wherein the electronegative atom holds a negative charge. On the other hand when an alkyl group is attached to a carbon chain then this is called +I effect.
Here in the option B carboxylic acid gives a proton and the anion which is left is stabilized by the –I effect and this shows that stronger will be the –I effect more will be stability and more will be the acidic strength. The presence of 2 chlorine atoms in option B shows the maximum –I effect and it has maximum acidic strength.
Thus option B is the correct answer.
Note:
Inductive Effect refers to the phenomenon by which a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds whereas the electromeric effect can only arise in pi bonds.
Complete answer:
When any electron releasing group or electron withdrawing group is attached with a chain of carbon atoms then partially positive or negative charge arises on the carbon chain atoms which causes permanent dipole in the molecule this type of effect is known as inductive effect. There are two types of inductive effect known as +I effect and –I effect.
-I effect occurs when an electronegative atom like halogen is attached to a chain of atoms resulting in unequal sharing of electrons which generates a positive charge. It causes a permanent dipole in the molecule wherein the electronegative atom holds a negative charge. On the other hand when an alkyl group is attached to a carbon chain then this is called +I effect.
Here in the option B carboxylic acid gives a proton and the anion which is left is stabilized by the –I effect and this shows that stronger will be the –I effect more will be stability and more will be the acidic strength. The presence of 2 chlorine atoms in option B shows the maximum –I effect and it has maximum acidic strength.
Thus option B is the correct answer.
Note:
Inductive Effect refers to the phenomenon by which a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds whereas the electromeric effect can only arise in pi bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




