
Schiff's reagent is used for the differentiation between :
Answer
590.4k+ views
Hint: Try to recall the Schiff's test given in aldehydes and ketones chapter of class XII . Schiff's reagent is used to distinguish between aldehydes and ketones. Ketones do not react with Schiff's reagent; however, aldehydes react with Schiff's reagent.
Complete answer:
The Schiff test is a chemical test used to check the presence of aldehydes in a solution. This is done by reacting the solution with a small quantity of Schiff's reagent.
Schiff's reagent is the reaction product of a dye such as fuchsin and sodium bisulfite.
Schiff's reagent is given in the below figure:
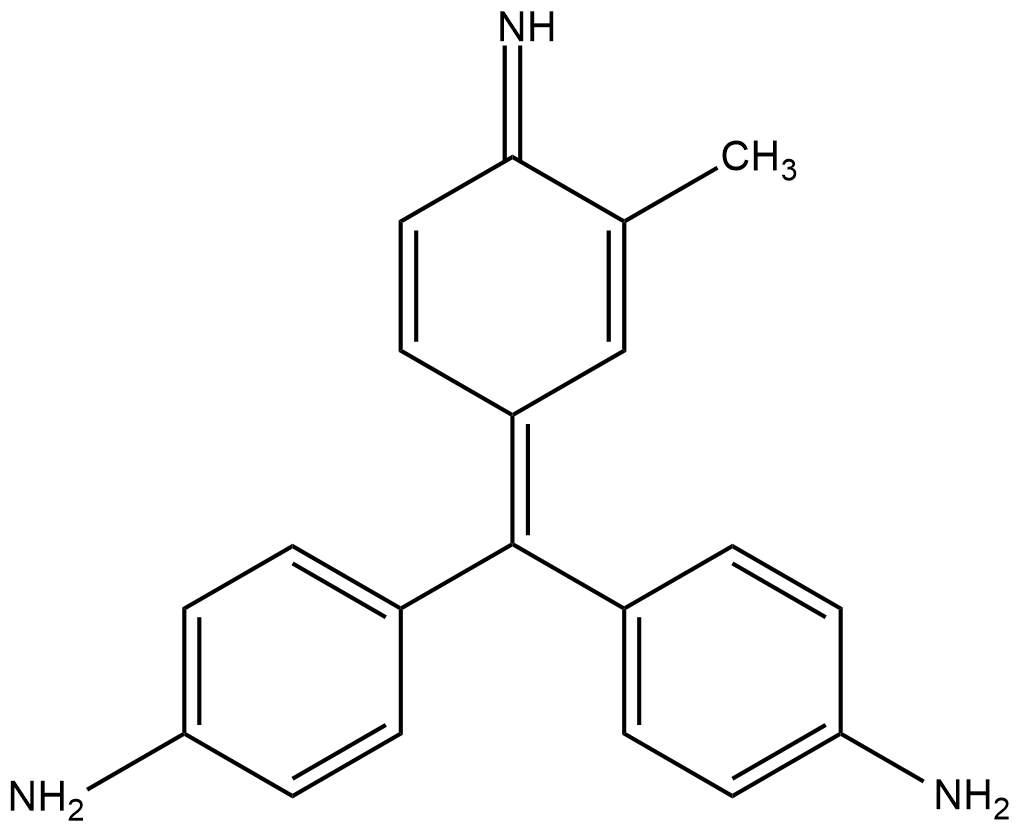
Reaction mechanism:
Fuchsin solutions appear colored as the wavelength of light absorbed lies in the visible range. In addition to bisulfite, a decolorized adduct is formed with the central carbon atom being sulfonated. This adduct is an excellent electrophile. When aldehyde is introduced, it readily reacts with aldehyde and further undergoes reaction with bisulfite ion to give a dark purple coloration to the solution.
Let us now see the options that have an aldehyde and ketone. This is because aldehyde will react with Schiff's reagent and ketones are inert to the reagent. Hence, we can distinguish between the two molecules.
We find that options (A),(B) as well as (D) have two aldehydes. Schiff's reagent cannot be used to distinguish between two aldehydes as both will give coloration. Option C has an aldehyde and a ketone.
Therefore, the correct answer is option (C).
Note:
The reaction mechanism explained in the solution is a widely accepted mechanism and there have been several attempts to prove the coloration is due to a different reaction happening between the aldehyde and Schiff's reagent. However, they have not been accepted as they cannot answer all the phenomena observed in the reaction.
Complete answer:
The Schiff test is a chemical test used to check the presence of aldehydes in a solution. This is done by reacting the solution with a small quantity of Schiff's reagent.
Schiff's reagent is the reaction product of a dye such as fuchsin and sodium bisulfite.
Schiff's reagent is given in the below figure:

Reaction mechanism:
Fuchsin solutions appear colored as the wavelength of light absorbed lies in the visible range. In addition to bisulfite, a decolorized adduct is formed with the central carbon atom being sulfonated. This adduct is an excellent electrophile. When aldehyde is introduced, it readily reacts with aldehyde and further undergoes reaction with bisulfite ion to give a dark purple coloration to the solution.
Let us now see the options that have an aldehyde and ketone. This is because aldehyde will react with Schiff's reagent and ketones are inert to the reagent. Hence, we can distinguish between the two molecules.
We find that options (A),(B) as well as (D) have two aldehydes. Schiff's reagent cannot be used to distinguish between two aldehydes as both will give coloration. Option C has an aldehyde and a ketone.
Therefore, the correct answer is option (C).
Note:
The reaction mechanism explained in the solution is a widely accepted mechanism and there have been several attempts to prove the coloration is due to a different reaction happening between the aldehyde and Schiff's reagent. However, they have not been accepted as they cannot answer all the phenomena observed in the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




