
Schemes 1 and 2 describe the conversion of P to Q and R to S, respectively. Scheme 3 describes the synthesis of T from Q and S. The total number of Br atoms in a molecule of T is __________

Answer
531.6k+ views
Hint: The given compound as P is aniline, so when it reacts with bromine water, then there will be the addition of three bromine atoms on it. In the next step, there will be diazotization. When the benzene reacts with oleum, then there is an addition of sulfate group on the benzene ring.
Complete answer:
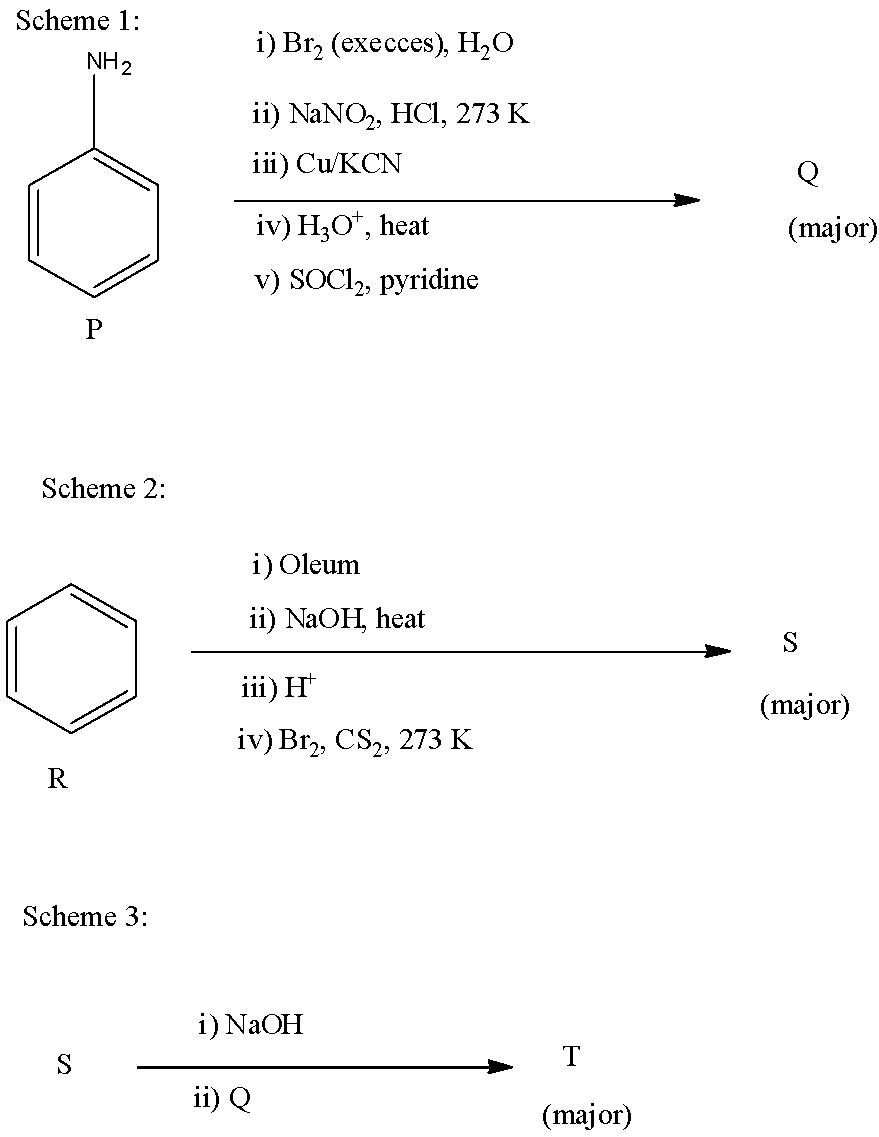
In scheme 1, the compound P is aniline, in the first step, it is treated with bromine water. There will be the addition of three bromine atoms, forming 2, 4, 6-tribromoaniline. 2, 4, 6-tribromoaniline, when reacted with $NaN{{O}_{2}}$ and HCl, there will be diazotization, further when it is reacted with copper cyanide or potassium cyanide, will replace the amine group with a cyano group. Now, when this is hydrolyzed, the cyano group converts into the carboxylic group. Now, this reaction on treatment with $SOC{{l}_{2}}$ and pyridine, the carboxylic acid converts into –COCl group. The reactions are given below:
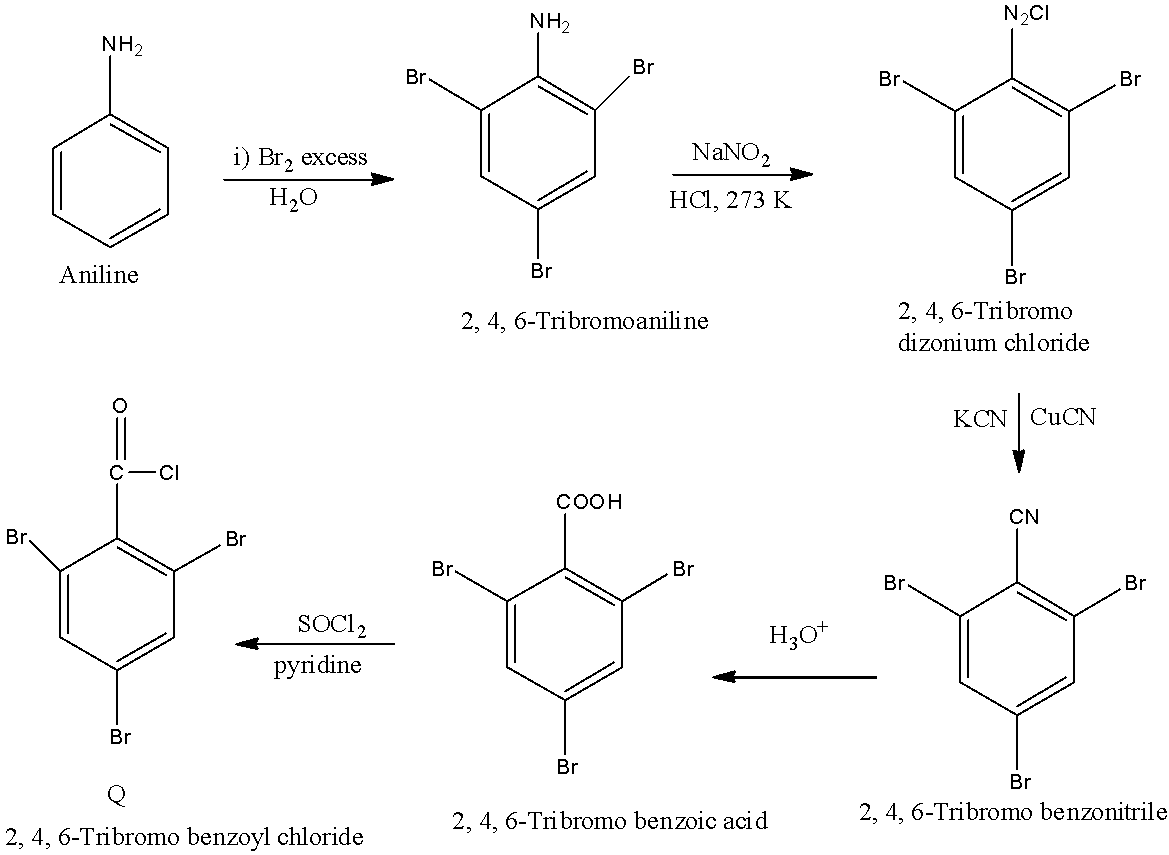
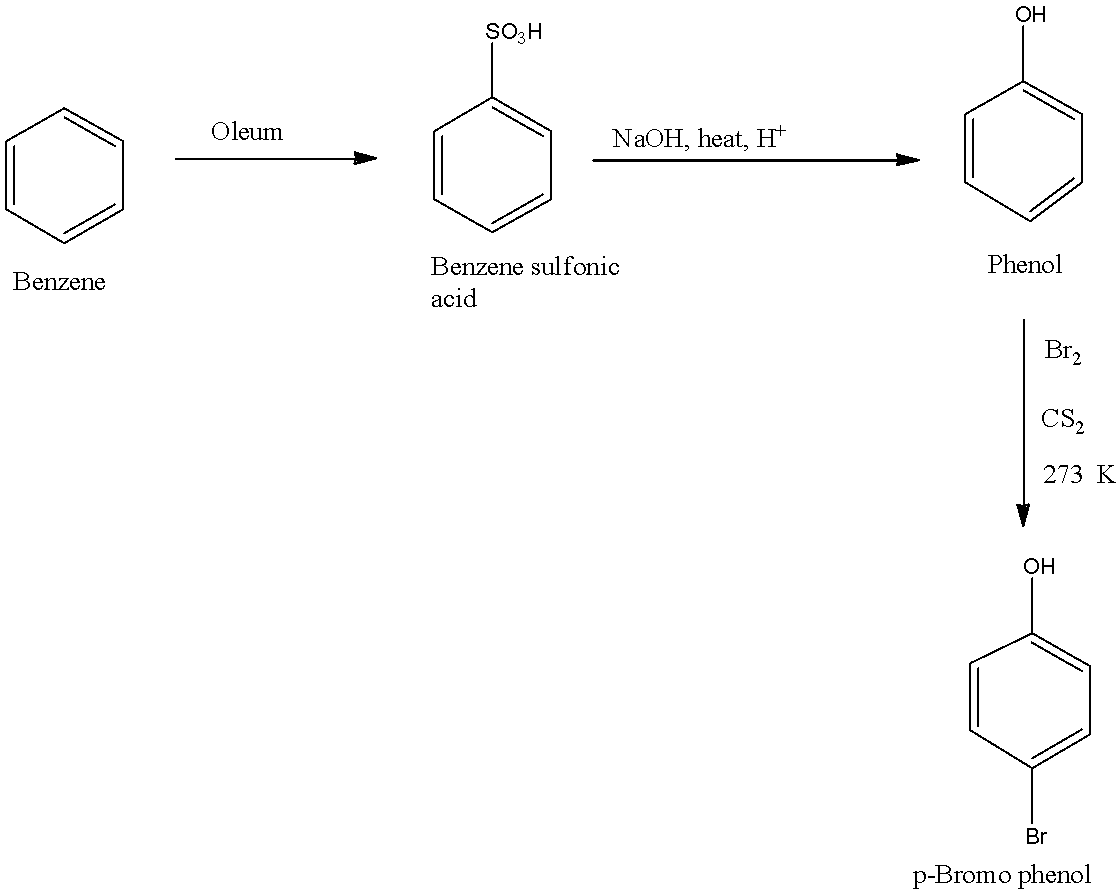
In scheme 2, when the benzene reacts with oleum, there will be the addition of sulfate group on the benzene ring. When this compound reacts with sodium hydroxide in the presence of an acid, the sulfate group is replaced with a hydroxyl group. This phenol reacts with bromine in the presence of carbon sulfide, there will be the addition of bromine atom on the ortho and para position, but the para position will be the major product. The reactions are given below:
In scheme 3, the S reacts with sodium hydroxide with Q to form T, the reaction will be:
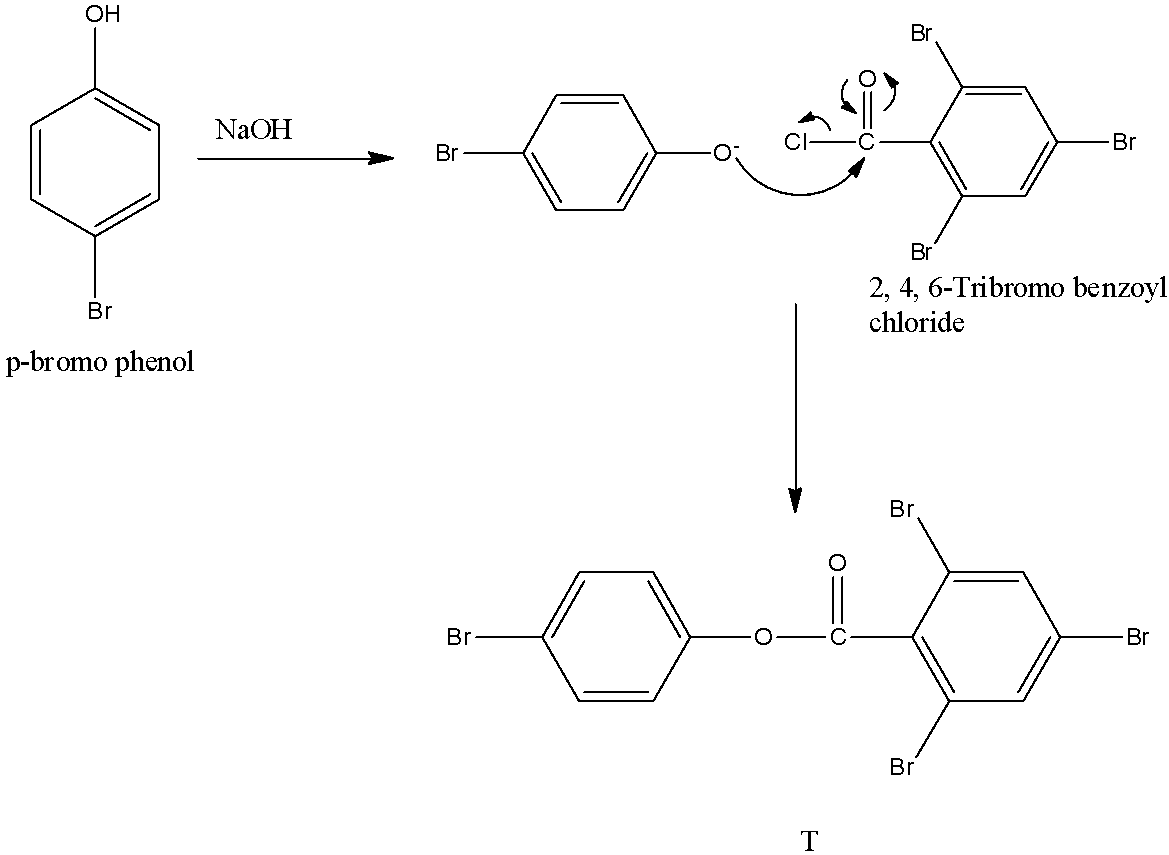
In T, there are 4 bromine atoms.
Note:
It must be noted that when the reaction is with bromine water, then there will be the addition of three bromine atoms, and when the reaction is with bromine in the presence of carbon sulfide, there will be the addition of only one bromine atom.
Complete answer:
In scheme 1, the compound P is aniline, in the first step, it is treated with bromine water. There will be the addition of three bromine atoms, forming 2, 4, 6-tribromoaniline. 2, 4, 6-tribromoaniline, when reacted with $NaN{{O}_{2}}$ and HCl, there will be diazotization, further when it is reacted with copper cyanide or potassium cyanide, will replace the amine group with a cyano group. Now, when this is hydrolyzed, the cyano group converts into the carboxylic group. Now, this reaction on treatment with $SOC{{l}_{2}}$ and pyridine, the carboxylic acid converts into –COCl group. The reactions are given below:

In scheme 2, when the benzene reacts with oleum, there will be the addition of sulfate group on the benzene ring. When this compound reacts with sodium hydroxide in the presence of an acid, the sulfate group is replaced with a hydroxyl group. This phenol reacts with bromine in the presence of carbon sulfide, there will be the addition of bromine atom on the ortho and para position, but the para position will be the major product. The reactions are given below:

In scheme 3, the S reacts with sodium hydroxide with Q to form T, the reaction will be:

In T, there are 4 bromine atoms.
Note:
It must be noted that when the reaction is with bromine water, then there will be the addition of three bromine atoms, and when the reaction is with bromine in the presence of carbon sulfide, there will be the addition of only one bromine atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




