
$Sb{F}_{5}$ reacts with $Xe{F}_{4}$ to form an adduct. The shapes of cation and anion in the adduct are respectively:
a.) square planar, trigonal bipyramidal
b.) T-shaped, octahedral
c.) square pyramidal, octahedral
d.) square planar, octahedral
Answer
597.9k+ views
Hint: Hybridization is the notion that newly hybridized orbitals are formed from the fusion of atomic orbitals. This in turn, influences the molecular bonding and geometry properties of the compound.
Complete answer:
The energy of the hybridized orbitals are lower than the energy compared to their
separated, unhybridized counterparts. Due to this more stable compounds are formed.
The hybridization also has a greater role in determining the shape or the molecular geometry of the compound. The shapes can be: linear, angular or V or bent, tetrahedral, seesaw, square pyramidal and many more.
Hybridization is an expansion of valence bond theory.
The Hybridization of any compound can be calculated as follows:
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ V+M-C+A \right]$ ----(1)
where,
H = Number of orbitals involved in hybridization.
V = Valence electrons of the central atom.
M = Number of monovalent atoms linked to the central atom.
C = Charge of the cation.
A = Charge of the anion.
Now, let us consider the question, the reaction involved is shown below.
$Sb{ F }_{ 5 }\quad +\quad Xe{ F }_{ 4 }\quad \longrightarrow \quad { [Xe{ F }_{ 3 }] }^{ + }{ [Sb{ F }_{ 6 }] }^{ - }$
In the case of ${[Xe{F}_{3}]}^{+}$, V=8, M=3 and C=1. Substituting these values in equation (1), we get,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ 8+5-1 \right]$
$\implies H = 5$
This shows that it has 3 bonded pairs and 2 lone pairs and has a $s{p}^{3}d$ hybridization and thus has a T-shaped structure.
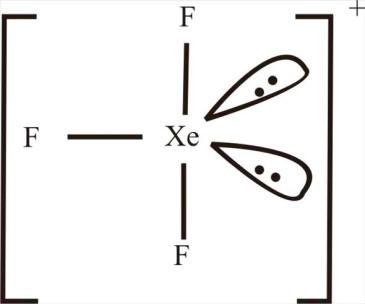
In the case of ${[Sb{F}_{6}]}^{-}$, V=5, M=6 and A=1. Substituting these values in equation (1), we get,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ 5+6+1 \right]$
$\implies H = 6$
This shows that it has 6 bonded pairs and has a $s{p}^{3}{d}^{2}$ hybridization and thus has an octahedral structure.
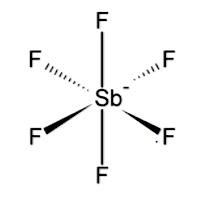
Note: It is due to the presence of lone pairs in ${[Xe{F}_{3}]}^{+}$ that it has a T-shaped structure and not a planar structure. It is due to the lone pair-lone pair repulsion that it has a T-shaped structure.
Complete answer:
The energy of the hybridized orbitals are lower than the energy compared to their
separated, unhybridized counterparts. Due to this more stable compounds are formed.
The hybridization also has a greater role in determining the shape or the molecular geometry of the compound. The shapes can be: linear, angular or V or bent, tetrahedral, seesaw, square pyramidal and many more.
Hybridization is an expansion of valence bond theory.
The Hybridization of any compound can be calculated as follows:
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ V+M-C+A \right]$ ----(1)
where,
H = Number of orbitals involved in hybridization.
V = Valence electrons of the central atom.
M = Number of monovalent atoms linked to the central atom.
C = Charge of the cation.
A = Charge of the anion.
Now, let us consider the question, the reaction involved is shown below.
$Sb{ F }_{ 5 }\quad +\quad Xe{ F }_{ 4 }\quad \longrightarrow \quad { [Xe{ F }_{ 3 }] }^{ + }{ [Sb{ F }_{ 6 }] }^{ - }$
In the case of ${[Xe{F}_{3}]}^{+}$, V=8, M=3 and C=1. Substituting these values in equation (1), we get,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ 8+5-1 \right]$
$\implies H = 5$
This shows that it has 3 bonded pairs and 2 lone pairs and has a $s{p}^{3}d$ hybridization and thus has a T-shaped structure.

In the case of ${[Sb{F}_{6}]}^{-}$, V=5, M=6 and A=1. Substituting these values in equation (1), we get,
$H\quad =\quad \cfrac { 1 }{ 2 } \left[ 5+6+1 \right]$
$\implies H = 6$
This shows that it has 6 bonded pairs and has a $s{p}^{3}{d}^{2}$ hybridization and thus has an octahedral structure.

Note: It is due to the presence of lone pairs in ${[Xe{F}_{3}]}^{+}$ that it has a T-shaped structure and not a planar structure. It is due to the lone pair-lone pair repulsion that it has a T-shaped structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




