
What is the same in various hydrides of chalcogens?
A.Molecular shape
B.Reducing nature
C.Central bond angle
D.Magnitude of interparticle forces
Answer
514.2k+ views
Hint: Now, before starting, we should know that chalcogens belong to which group. Chalcogens are a group of elements which belong to group-\[16\]. Group-\[16\] contains elements like oxygen \[(O)\], selenium \[(Se)\], tellurium\[(Te)\], etc.
Complete answer: Hydrides of chalcogens are the compounds having hydrogen and chalcogens atoms together. For example: Water molecule- formula of water molecule is ${H_2}O$, it contains two atoms of Hydrogen and one atom of oxygen, oxygen is an element which is present in Group-\[16\]only and is known as chalcogens.
Now, let’s see these options one by one.
Magnitude of interparticle forces: Magnitude of interparticle forces are not the same in the hydrides of chalcogens. Considerable magnitude of interparticle force is of water molecule, that is, ${H_2}O$ other than that all the hydrides of the chalcogens have weak intermolecular forces.
Central bond angle: It is, also, not same for all the hydrides. Central bond angle of $H - O - H$is close to the tetrahedral angle which is $109.5^\circ $. $H - O - H$has a central bond angle of $104.45^\circ $. Other than that, $H - E - H$has a central bond angle very less than the tetrahedral angle, they are all about $90^\circ $, which is very less than the tetrahedral angle.
Reducing nature: All the hydrides of group- \[16\]are reducing agents except for the hydride of oxygen, that is, water molecules. Other than that all are the reducing agent and in group-\[16\] the reducing power increases down the group due to the increase in their atomic size and also due to the decrease in their Metal- Hydrogen bonding.
Molecular Shape: Each of the hydrides of the chalcogens have the same shape, that is, they all have V-shape or bent shape. For example: let’s see water molecule-
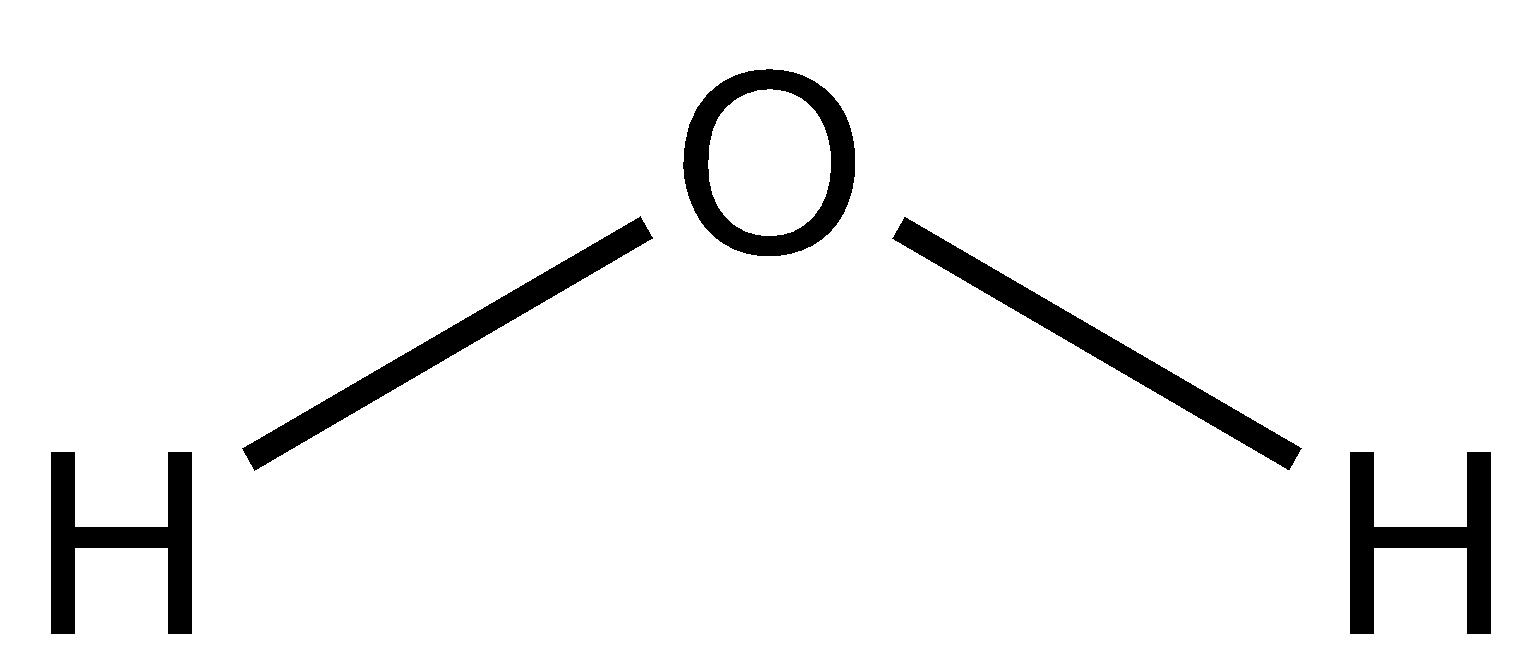
Similarly, other hydrides of the chalcogens also have a V or bent shape.
Therefore, the correct option is- Option A.
Note:
In the periodic table each group has their own specific chemical and physical properties. Chalcogens have several applications as well such as they are used in steelmaking, water treatment, rocket fuel, etc. It has several biological roles as well, for example- oxygen, we all know that we need oxygen to live, to breath. Oxygen is also a chalcogen.
Complete answer: Hydrides of chalcogens are the compounds having hydrogen and chalcogens atoms together. For example: Water molecule- formula of water molecule is ${H_2}O$, it contains two atoms of Hydrogen and one atom of oxygen, oxygen is an element which is present in Group-\[16\]only and is known as chalcogens.
Now, let’s see these options one by one.
Magnitude of interparticle forces: Magnitude of interparticle forces are not the same in the hydrides of chalcogens. Considerable magnitude of interparticle force is of water molecule, that is, ${H_2}O$ other than that all the hydrides of the chalcogens have weak intermolecular forces.
Central bond angle: It is, also, not same for all the hydrides. Central bond angle of $H - O - H$is close to the tetrahedral angle which is $109.5^\circ $. $H - O - H$has a central bond angle of $104.45^\circ $. Other than that, $H - E - H$has a central bond angle very less than the tetrahedral angle, they are all about $90^\circ $, which is very less than the tetrahedral angle.
Reducing nature: All the hydrides of group- \[16\]are reducing agents except for the hydride of oxygen, that is, water molecules. Other than that all are the reducing agent and in group-\[16\] the reducing power increases down the group due to the increase in their atomic size and also due to the decrease in their Metal- Hydrogen bonding.
Molecular Shape: Each of the hydrides of the chalcogens have the same shape, that is, they all have V-shape or bent shape. For example: let’s see water molecule-

Similarly, other hydrides of the chalcogens also have a V or bent shape.
Therefore, the correct option is- Option A.
Note:
In the periodic table each group has their own specific chemical and physical properties. Chalcogens have several applications as well such as they are used in steelmaking, water treatment, rocket fuel, etc. It has several biological roles as well, for example- oxygen, we all know that we need oxygen to live, to breath. Oxygen is also a chalcogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




