
How is salicylic acid converted into aspirin? Give an equation.
Answer
578.1k+ views
Hint: Aspirin is mainly a drug dosage used in a fever or as a painkiller. This is the most commonly used drug or we can say most commonly used pain killer. Aspirin is the common name for the compound acetylsalicylic acid. It is generally derived from salicylic acid.
Complete answer:
Generally the name of salicylic acid comes from the word Salix which represents the willow family of plants which was derived from willow bark extracts. In folk medicines willow bark trees were used as headache remedies and as other tonics too. But these create irritations in the stomach therefore, nowadays salicylic acid is administered in the form of aspirin which is less irritating to the stomach.
For the preparation of aspirin, salicylic acid is reacted with an excess of acetic anhydride. Small amount of strong acid is used as a catalyst in these reactions which enhances the speed of reaction acid used is phosphoric acid. Excess acetic acid will be extinguished with the addition of water. Aspirin product is not soluble in water so it forms precipitate when water is added and the reaction of synthesis can be shown as:
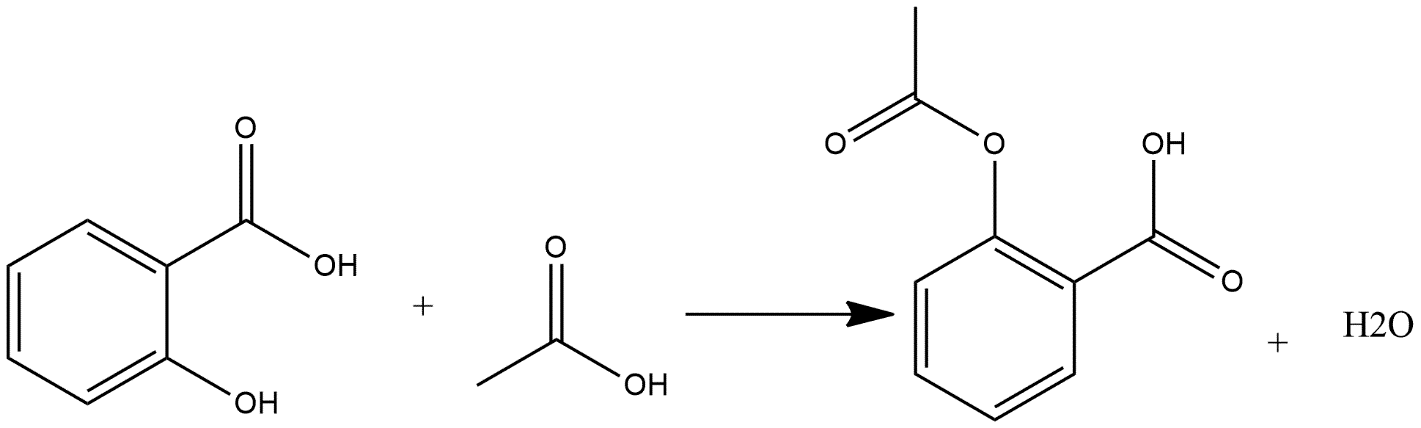
Note:
Aspirin decomposes rapidly in solutions of ammonium acetate or carbonates, citrates or hydroxides of the alkali metals. It is stable in dry air but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate.
Complete answer:
Generally the name of salicylic acid comes from the word Salix which represents the willow family of plants which was derived from willow bark extracts. In folk medicines willow bark trees were used as headache remedies and as other tonics too. But these create irritations in the stomach therefore, nowadays salicylic acid is administered in the form of aspirin which is less irritating to the stomach.
For the preparation of aspirin, salicylic acid is reacted with an excess of acetic anhydride. Small amount of strong acid is used as a catalyst in these reactions which enhances the speed of reaction acid used is phosphoric acid. Excess acetic acid will be extinguished with the addition of water. Aspirin product is not soluble in water so it forms precipitate when water is added and the reaction of synthesis can be shown as:

Note:
Aspirin decomposes rapidly in solutions of ammonium acetate or carbonates, citrates or hydroxides of the alkali metals. It is stable in dry air but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




