
How many $S - S$ linkage(s) is/are present in sodium tetrathionate?
A. 4
B. 3
C. 2
D. 1
Answer
559.2k+ views
Hint: Sodium tetrathionate is composed of elements sodium (\[Na\]), sulphur (\[S\]), oxygen (\[O\]) and hydrogen (\[H\]). The chemical formula of sodium tetrathionate salt is $N{a_2}{S_4}{O_6}x{H_2}O$. The compound is also known as sodium (sulfonato de sulfanyl) sulfonate dihydrate. It is categorised as polythionate. It is colourless solid and is soluble in water.
Complete step by step answer:
Here, we have to identify the correct number of $S - S$ linkages or bonds in sodium tetrathionate. The $S - S$ linkages here refers to the bonds present between S atoms present in sodium tetrathionate. The sulphur is one of the elements present in sodium tetrathionate, along with sodium, oxygen and hydrogen. From the chemical formula of sodium tetrathionate, we can say that there are 4 sulphur atoms present in the compound.
To identify the number of $S - S$ linkages correctly, we first need to draw the structure of the compound and then count the number of $S - S$ linkages in it.
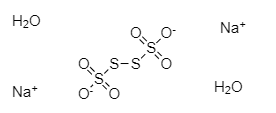
As we can see in the above structure, two sulphur atoms are linked to three oxygen atoms each and remaining two sulphur atoms are bonded to other two sulphur atoms. The two sulphur atoms in the centre are also bonded to each other. There are two sodium ions in the compound and two oxygen atoms are carrying negative charge. There are two water molecules as this is a dihydrate compound.
The four sulphur atoms are bonded to each other through three $S - S$ bonds or linkages.
So, the correct answer is Option B.
Additional information:
The compound sodium tetrathionate is formed by the action of iodine on sodium thiosulphate. This is an oxidation reaction.
The chemical reaction for the same is given below.
$2N{a_2}{S_2}{O_3} + {I_2} \to N{a_2}{S_4}{O_6} + 2NaI$
The discoloration of iodine signifies that oxidation of sodium thiosulphate is carried out.
Note: As we know, the compound sodium tetrathionate contains four sulphur atoms. Remember that, the word ‘thio’ denotes that the compound contains sulphur as one of its constituent elements. It also indicates that the compound is derived from another compound containing oxygen atoms and these oxygen atoms then replaced by sulphur atoms. The word ‘tetra’ in the name of the compound suggests that there are 4 oxygen atoms were replaced by sulphur atoms and this compound contains 4 sulphur atoms.
Complete step by step answer:
Here, we have to identify the correct number of $S - S$ linkages or bonds in sodium tetrathionate. The $S - S$ linkages here refers to the bonds present between S atoms present in sodium tetrathionate. The sulphur is one of the elements present in sodium tetrathionate, along with sodium, oxygen and hydrogen. From the chemical formula of sodium tetrathionate, we can say that there are 4 sulphur atoms present in the compound.
To identify the number of $S - S$ linkages correctly, we first need to draw the structure of the compound and then count the number of $S - S$ linkages in it.

As we can see in the above structure, two sulphur atoms are linked to three oxygen atoms each and remaining two sulphur atoms are bonded to other two sulphur atoms. The two sulphur atoms in the centre are also bonded to each other. There are two sodium ions in the compound and two oxygen atoms are carrying negative charge. There are two water molecules as this is a dihydrate compound.
The four sulphur atoms are bonded to each other through three $S - S$ bonds or linkages.
So, the correct answer is Option B.
Additional information:
The compound sodium tetrathionate is formed by the action of iodine on sodium thiosulphate. This is an oxidation reaction.
The chemical reaction for the same is given below.
$2N{a_2}{S_2}{O_3} + {I_2} \to N{a_2}{S_4}{O_6} + 2NaI$
The discoloration of iodine signifies that oxidation of sodium thiosulphate is carried out.
Note: As we know, the compound sodium tetrathionate contains four sulphur atoms. Remember that, the word ‘thio’ denotes that the compound contains sulphur as one of its constituent elements. It also indicates that the compound is derived from another compound containing oxygen atoms and these oxygen atoms then replaced by sulphur atoms. The word ‘tetra’ in the name of the compound suggests that there are 4 oxygen atoms were replaced by sulphur atoms and this compound contains 4 sulphur atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




