
Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane is:
1. ${{360}^{\circ }}$
2. ${{180}^{\circ }}$
3. ${{120}^{\circ }}$
4. ${{240}^{\circ }}$
Answer
564.9k+ views
Hint: As we know that different conformations are the arrangement of two atoms in a molecule that are found to differ by rotation. And the study of energy between different conformations is called conformational analysis.
Complete Solution :
- By rotating the back carbon ${{C}_{2}}-{{C}_{3}}$ about axis by an angle of ${{180}^{\circ }}$, the resulting conformation is called as Anti conformation or staggered conformation.
- We can see from the conformation drawn below:
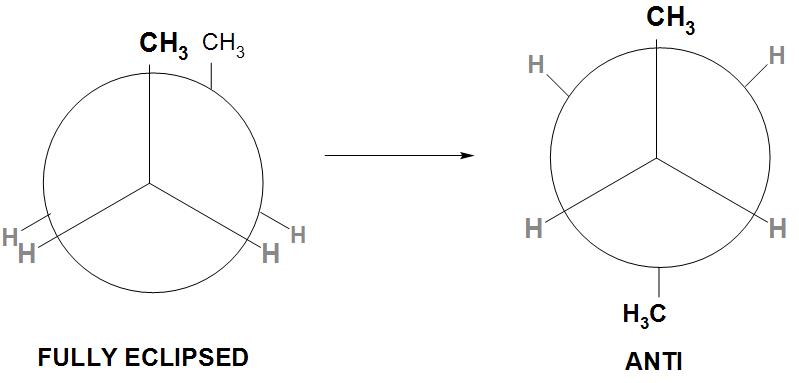
- We can see from the above staggered conformation that all the groups on ${{C}_{2}}$ and ${{C}_{3}}$ carbons are maximum apart. Therefore, it is found that the torsional and the steric interactions are minimum. ( the bulkier two$C{{H}_{3}}$ groups are at ${{180}^{\circ }}$).
- Hence, it is found that this is the most stable and the most preferred conformation of n-butane. Therefore, its potential energy is considered as zero and the energies of all other conformation is determined relative to this.
- Hence, we can say that the correct option is (1), that is the Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane is ${{180}^{\circ }}$
So, the correct answer is “Option 2”.
Note: - We should note the main difference in between eclipsed and staggered conformation. In eclipsed conformation, the carbons are aligned in such a way that the hydrogens are lined up with each other.
- In staggered conformation atoms are found to be equally spaced from each other.
Complete Solution :
- By rotating the back carbon ${{C}_{2}}-{{C}_{3}}$ about axis by an angle of ${{180}^{\circ }}$, the resulting conformation is called as Anti conformation or staggered conformation.
- We can see from the conformation drawn below:

- We can see from the above staggered conformation that all the groups on ${{C}_{2}}$ and ${{C}_{3}}$ carbons are maximum apart. Therefore, it is found that the torsional and the steric interactions are minimum. ( the bulkier two$C{{H}_{3}}$ groups are at ${{180}^{\circ }}$).
- Hence, it is found that this is the most stable and the most preferred conformation of n-butane. Therefore, its potential energy is considered as zero and the energies of all other conformation is determined relative to this.
- Hence, we can say that the correct option is (1), that is the Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane is ${{180}^{\circ }}$
So, the correct answer is “Option 2”.
Note: - We should note the main difference in between eclipsed and staggered conformation. In eclipsed conformation, the carbons are aligned in such a way that the hydrogens are lined up with each other.
- In staggered conformation atoms are found to be equally spaced from each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




