
What is the role of pyridine in the acylation of amines?
Answer
519.9k+ views
Hint :in the given question we should know about the chemical reaction of the acylation of amines. When the acyl group is added to a molecule, that type of chemical reaction is called an acylation reaction. Amines are the organic compounds which contain a nitrogen atom with a lone pair of electrons. Acylation of amines is the reaction of amines with the acetyl chloride.
Complete Step By Step Answer:
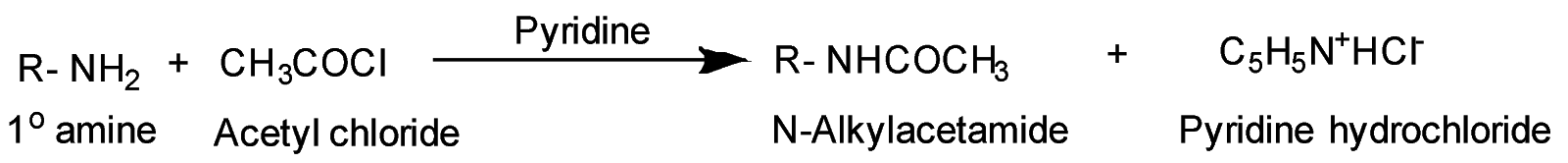
Acylation of amines is the reaction between the amines ( $ - N{H_2} $ group) and the acetyl group ( $ C{H_3}CO - $ ) that comes from either acetyl halide or acetic anhydride. This reaction takes place in the presence of pyridine ( $ {C_5}{H_5}N $ ) which acts as a strong base during the reaction. During substitution reaction of acylation of ( $ - N{H_2} $ ) group with acetyl group followed by hydrolysis of substituted amide with substituted amine, the activating effect of $ - N{H_2} $ group can be controlled. Pyridine is used to remove the side product formed in the acylation reaction i.e. $ HCl $ from the reaction mixture. It acts as an acceptor for the acid byproduct formed in the reaction.
Pyridine also acts as a nucleophile for the carbonyl group due to its lone pair of electrons on nitrogen atoms which can be delocalized in its ring. It acts as a catalyst and is often used in the acylation reactions.
Structure of Pyridine-
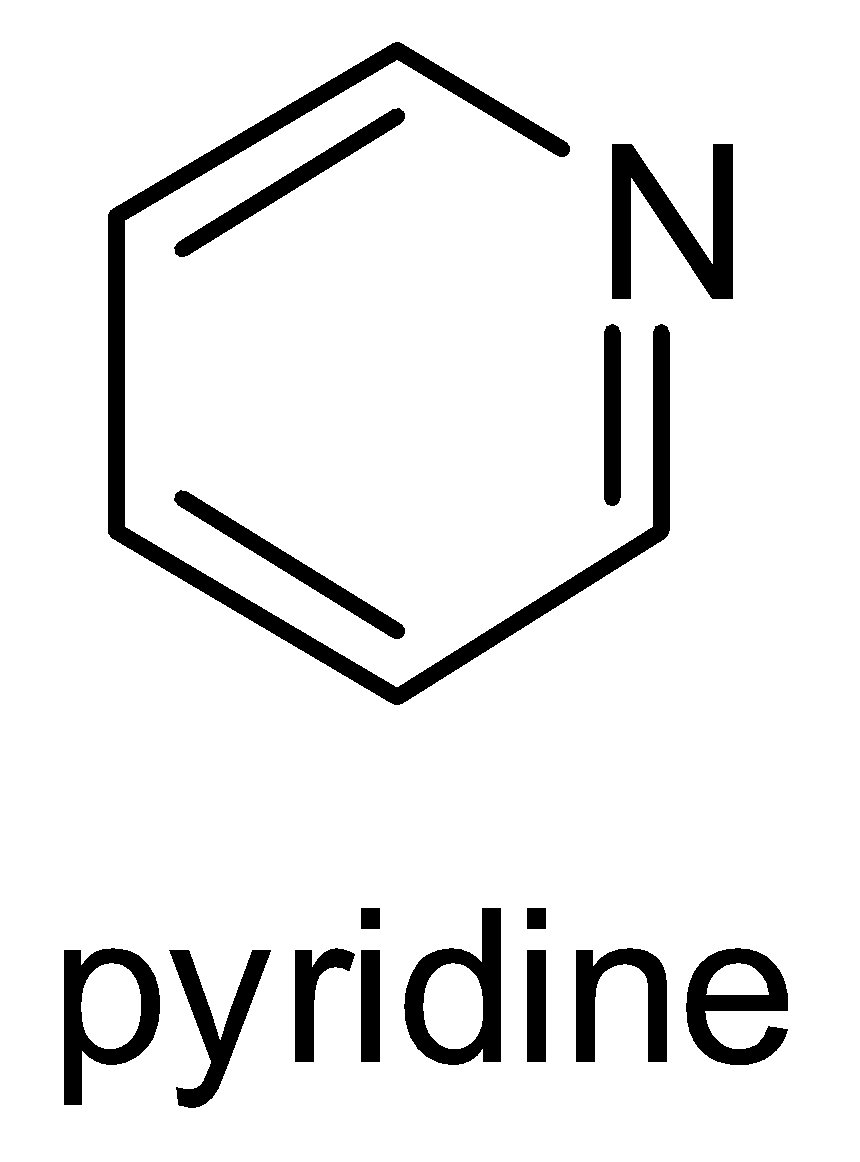
Example- Chemical Reaction between primary amine and acetyl chloride
Note :
It needs to be remembered that pyridine is a basic heterocyclic organic compound that acts as a base as well as nucleophile. It has a lone pair of electrons on nitrogen atoms which get delocalized in the ring. Structure of pyridine and its molecular formula ( $ {C_5}{H_5}N $ ) should be remembered. Pyridine is a water- miscible liquid with a distinctive and unpleasant fish like smell. It is a highly flammable and weakly alkaline liquid.
Complete Step By Step Answer:
Acylation of amines is the reaction between the amines ( $ - N{H_2} $ group) and the acetyl group ( $ C{H_3}CO - $ ) that comes from either acetyl halide or acetic anhydride. This reaction takes place in the presence of pyridine ( $ {C_5}{H_5}N $ ) which acts as a strong base during the reaction. During substitution reaction of acylation of ( $ - N{H_2} $ ) group with acetyl group followed by hydrolysis of substituted amide with substituted amine, the activating effect of $ - N{H_2} $ group can be controlled. Pyridine is used to remove the side product formed in the acylation reaction i.e. $ HCl $ from the reaction mixture. It acts as an acceptor for the acid byproduct formed in the reaction.
Pyridine also acts as a nucleophile for the carbonyl group due to its lone pair of electrons on nitrogen atoms which can be delocalized in its ring. It acts as a catalyst and is often used in the acylation reactions.
Structure of Pyridine-

Example- Chemical Reaction between primary amine and acetyl chloride

Note :
It needs to be remembered that pyridine is a basic heterocyclic organic compound that acts as a base as well as nucleophile. It has a lone pair of electrons on nitrogen atoms which get delocalized in the ring. Structure of pyridine and its molecular formula ( $ {C_5}{H_5}N $ ) should be remembered. Pyridine is a water- miscible liquid with a distinctive and unpleasant fish like smell. It is a highly flammable and weakly alkaline liquid.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




