
Roasting of sulphides gives the gas X as a product. This is a colorless gas with a cooking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic and acts as a reducing agent and its acid has never been isolated. The gas X is:
A.${{H}_{2}}S$
B.$S{{O}_{2}}$
C.$C{{O}_{2}}$
D.$S{{O}_{3}}$
Answer
576.6k+ views
Hint:. This is a colourless gas with a choking smell of burnt sulphur and causes great damage to the respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated.
Complete step by step answer:
Roasting of sulphides gives us the gas $S{{O}_{2}}$ as a byproduct. This is a colorless gas that has a cooking smell of burnt sulphur and causes great damage to respiratory organs as a result of the acid rain. Its aqueous solution is acidic and does act like a reducing agent and its acid has never been isolated.
For example sulphur dioxide gas is obtained by roasting zinc blende or iron pyrites.
\[2ZnS+3{{O}_{2}}\text{ }\to 2ZnO+2S{{O}_{2}}\]
\[4Fe{{S}_{2}}\text{ }+11{{O}_{2}}\text{ }\to 2F{{e}_{2}}{{O}_{3}}\text{ }+8S{{O}_{2}}\]
Sulphur dioxide dissolves in water forming sulphurous acid. hence, it is known as sulphurous anhydride.
\[{{H}_{2}}O+S{{O}_{2}}={{H}_{2}}S{{O}_{3}}\]
It reduces chlorine to HCl.
\[S{{O}_{2}}+C{{l}_{2}}\text{ }+2{{H}_{2}}O\to {{H}_{2}}S{{O}_{4}}\text{ }+2HCl\]
Therefore it is $S{{O}_{2}}$ gas.
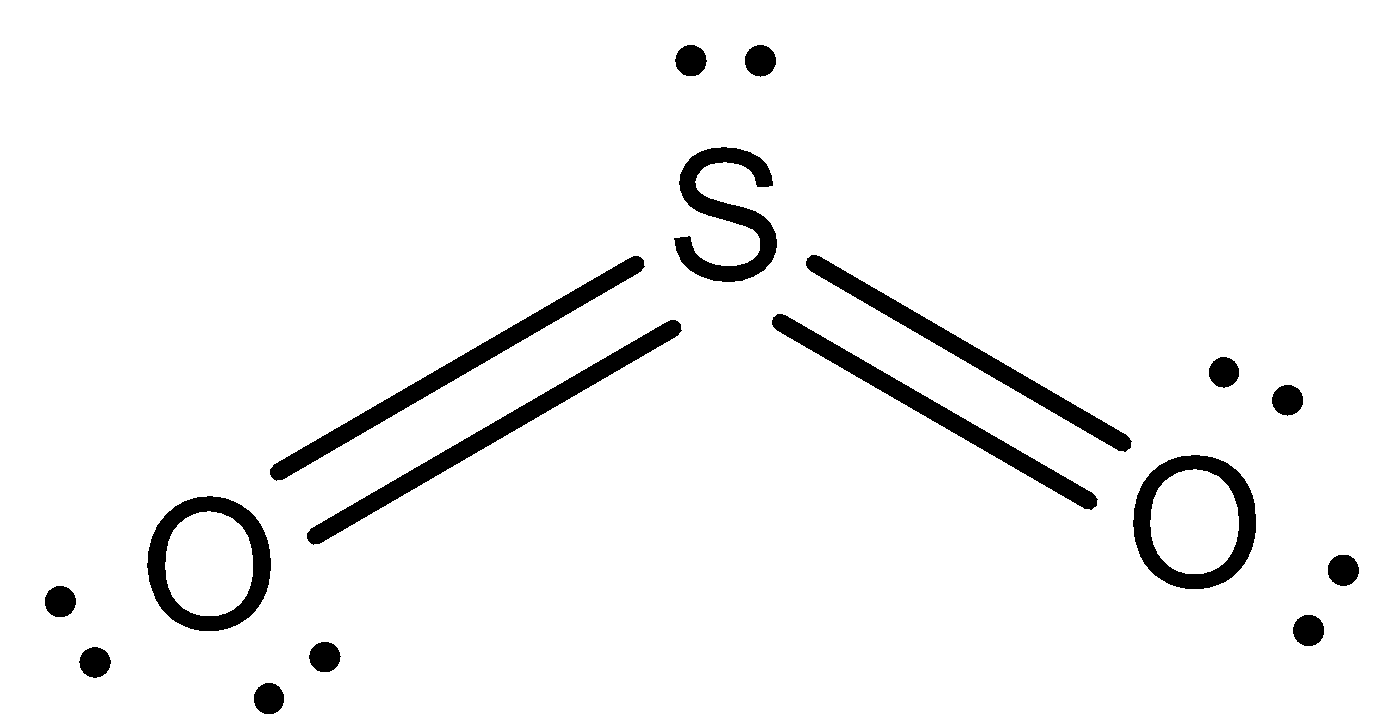
So, the correct answer is “Option B”.
Note: Sulfur dioxide or sulphur dioxide is the chemical compound with the formula $S{{O}_{2}}$. It is a very toxic gas which is the reason for the smell of burnt matches. It is released naturally by volcanic eruptions and is also produced as a by-product of copper extraction and the burning of fossil fuels is also contaminated with a good percentage of sulfur compounds. It is to be remembered that $S{{O}_{2}}$ is not a greenhouse gas, so it does not help in protection of the environment.
Molar mass of the gas is \[64.066\text{ }gmo{{l}^{-1}}\]
IUPAC name is Sulfur dioxide
Melting point: of the gas is ${{72}^{0}}C$
Boiling point of the gas is $-{{10}^{0}}C$
Complete step by step answer:
Roasting of sulphides gives us the gas $S{{O}_{2}}$ as a byproduct. This is a colorless gas that has a cooking smell of burnt sulphur and causes great damage to respiratory organs as a result of the acid rain. Its aqueous solution is acidic and does act like a reducing agent and its acid has never been isolated.
For example sulphur dioxide gas is obtained by roasting zinc blende or iron pyrites.
\[2ZnS+3{{O}_{2}}\text{ }\to 2ZnO+2S{{O}_{2}}\]
\[4Fe{{S}_{2}}\text{ }+11{{O}_{2}}\text{ }\to 2F{{e}_{2}}{{O}_{3}}\text{ }+8S{{O}_{2}}\]
Sulphur dioxide dissolves in water forming sulphurous acid. hence, it is known as sulphurous anhydride.
\[{{H}_{2}}O+S{{O}_{2}}={{H}_{2}}S{{O}_{3}}\]
It reduces chlorine to HCl.
\[S{{O}_{2}}+C{{l}_{2}}\text{ }+2{{H}_{2}}O\to {{H}_{2}}S{{O}_{4}}\text{ }+2HCl\]
Therefore it is $S{{O}_{2}}$ gas.

So, the correct answer is “Option B”.
Note: Sulfur dioxide or sulphur dioxide is the chemical compound with the formula $S{{O}_{2}}$. It is a very toxic gas which is the reason for the smell of burnt matches. It is released naturally by volcanic eruptions and is also produced as a by-product of copper extraction and the burning of fossil fuels is also contaminated with a good percentage of sulfur compounds. It is to be remembered that $S{{O}_{2}}$ is not a greenhouse gas, so it does not help in protection of the environment.
Molar mass of the gas is \[64.066\text{ }gmo{{l}^{-1}}\]
IUPAC name is Sulfur dioxide
Melting point: of the gas is ${{72}^{0}}C$
Boiling point of the gas is $-{{10}^{0}}C$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




