
${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CH = C}}{{\rm{H}}_{\rm{2}}}$${\rm{ + }}$${\rm{HBr}}$ gives
(A)${\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{ = CH}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{Br}}$
(B)${\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{Br}}$
(C)${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{CH(Br)}} - {\rm{C}}{{\rm{H}}_{\rm{3}}}$
(D)${\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{C}}{{\rm{H}}_{\rm{3}}}$
Answer
585.9k+ views
Hint:When an additional reaction involves initially the attack by an electrophile, the reaction is referred to as electrophilic addition. Compounds containing carbon-carbon double bond and triple bond undergo such reactions. The addition of ${\rm{HBr}}$to the propene is a common example of electrophilic addition.
Complete step by step answer:
In the addition of hydrogen bromide to a carbon-carbon double bond, proton is the electrophile and it forms a carbonium ion. Here two alternative carbonium ions are possible, which are secondary carbonium ions and primary carbonium ions.
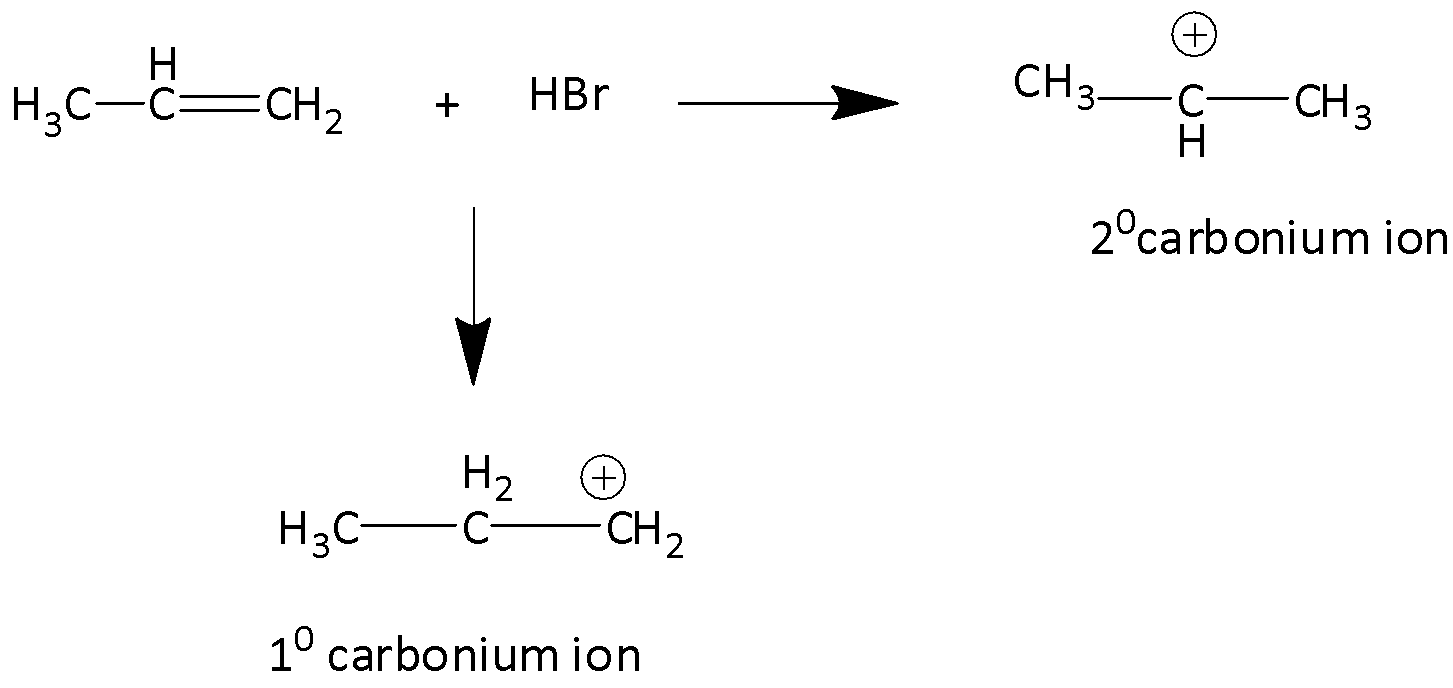
We know that the tertiary carbocation is more stable than secondary carbocation. Secondary carbocation in turn is more stable than primary carbocation. Therefore, isopropyl bromide is obtained as a major product whereas 1-Bromoprorpane is the minor product.
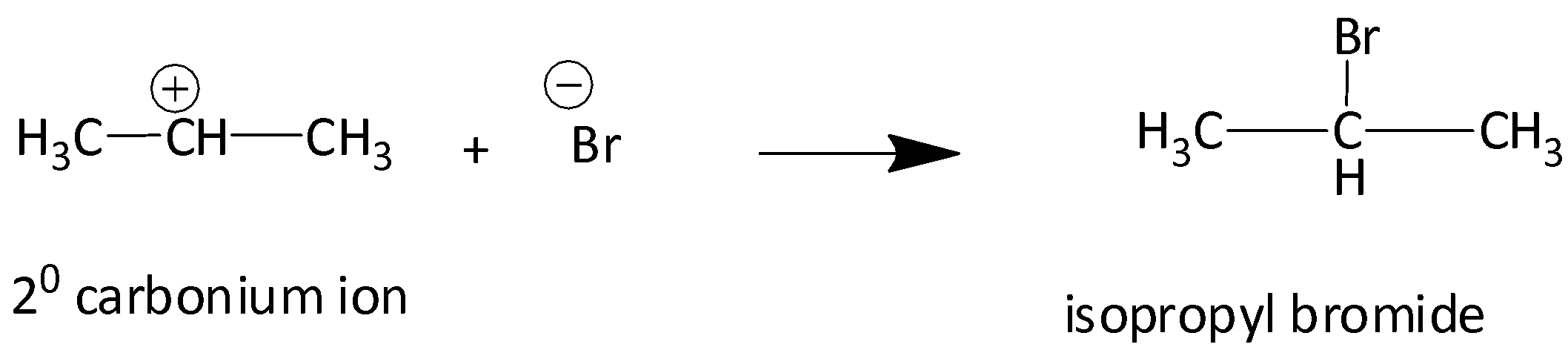
Therefore, according to Markonikov’s rule the correct option is C.
Additional information:
In propene, for instance, the inductive effect of the methyl group will stabilize the positive ionic charge on the carbon atom to which it is attached, so that the nucleophile will preferably attack on that very carbon. This explains what is known as Markonikov’s rule. When it was formulated Markonikov’s rule was purely empirical. However now a theoretical explanation of orientation of addition to olefins can be offered on the basis of relative stability of carbocation. If there is a probability of formation of more than one carbocation the addition of electrophile yields the more stable one.
Addition reactions are those in which elimination of atoms or other molecules does not take place, atoms or groups of atoms are simply added to a double or triple bond. This reaction can be initiated by electrophile, nucleophile or free radicals.
Note:
Markonikov’s rule depends upon the stability of the intermediate carbocations, that is the energies of the transition state are involved. According to this rule, where the hydrogen is added to the carbon with more hydrogen atoms, we will be the major product.
Complete step by step answer:
In the addition of hydrogen bromide to a carbon-carbon double bond, proton is the electrophile and it forms a carbonium ion. Here two alternative carbonium ions are possible, which are secondary carbonium ions and primary carbonium ions.

We know that the tertiary carbocation is more stable than secondary carbocation. Secondary carbocation in turn is more stable than primary carbocation. Therefore, isopropyl bromide is obtained as a major product whereas 1-Bromoprorpane is the minor product.

Therefore, according to Markonikov’s rule the correct option is C.
Additional information:
In propene, for instance, the inductive effect of the methyl group will stabilize the positive ionic charge on the carbon atom to which it is attached, so that the nucleophile will preferably attack on that very carbon. This explains what is known as Markonikov’s rule. When it was formulated Markonikov’s rule was purely empirical. However now a theoretical explanation of orientation of addition to olefins can be offered on the basis of relative stability of carbocation. If there is a probability of formation of more than one carbocation the addition of electrophile yields the more stable one.
Addition reactions are those in which elimination of atoms or other molecules does not take place, atoms or groups of atoms are simply added to a double or triple bond. This reaction can be initiated by electrophile, nucleophile or free radicals.
Note:
Markonikov’s rule depends upon the stability of the intermediate carbocations, that is the energies of the transition state are involved. According to this rule, where the hydrogen is added to the carbon with more hydrogen atoms, we will be the major product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




