
Ribulose is a pentose sugar.
(A) True
(B) False
Answer
597.6k+ views
Hint: A pentose sugar is a monosaccharide with 5 carbon atoms present in it. With this in mind, analyse the structure of Ribose.
Complete step-by-Step Solution:
Let us analyse Ribulose and analyse all its properties before coming up with a definitive answer.
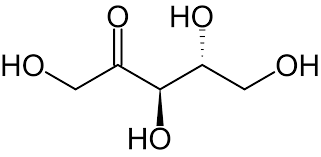
> Ribulose is a monosaccharide i.e. a singular carbohydrate molecule and contains five atoms of Carbon all of which sandwich a ketone functional group in its structure and its chemical formula is \[{{C}_{5}}{{H}_{10}}{{O}_{5}}\].
> Ribulose is an incredibly important biomolecule and is seen in different forms across many biological processes, for instance Ribulose 1,5-bisphosphate (RuBP) is a sugar that is very commonly seen in the process of photosynthesis.
Let us now look at the definition of pentoses and see whether Ribulose fits the criteria or not.
> In chemistry, a pentose is a monosaccharide (simple sugar) with five carbon atoms. The chemical formula of all pentoses is \[{{C}_{5}}{{H}_{10}}{{O}_{5}}\], and their molecular weight is 150.13 g/mol. Like some other monosaccharides, pentoses exist in two forms, open-chain (linear) or closed-chain (cyclic), that easily convert into each other in water solutions. The linear form of a pentose, which usually exists only in solutions, has an open-chain backbone of five carbons. Four of these carbons have one hydroxyl functional group (–OH) each, connected by a single bond, and one has an oxygen atom connected by a double bond (=O), forming a carbonyl group (C=O). The remaining bonds of the carbon atoms are satisfied by six hydrogen atoms. Thus, the structure of the linear form is \[H{{\left( CHOH \right)}_{x}}C\left( =O \right){{\left( CHOH \right)}_{4-x}}H\], where x is 0, 1, or 2.
Since Ribulose satisfies all of these criteria for pentose sugars, therefore the answer to this question is a) True.
Note: Unlike most sugars, ribulose does not exist as a mixture of cyclic forms in equilibrium with its linear form which readily interconvert especially in aqueous solution and only possesses one linear form. The structure of ribulose has two possible enantiomers.
Complete step-by-Step Solution:
Let us analyse Ribulose and analyse all its properties before coming up with a definitive answer.

> Ribulose is a monosaccharide i.e. a singular carbohydrate molecule and contains five atoms of Carbon all of which sandwich a ketone functional group in its structure and its chemical formula is \[{{C}_{5}}{{H}_{10}}{{O}_{5}}\].
> Ribulose is an incredibly important biomolecule and is seen in different forms across many biological processes, for instance Ribulose 1,5-bisphosphate (RuBP) is a sugar that is very commonly seen in the process of photosynthesis.
Let us now look at the definition of pentoses and see whether Ribulose fits the criteria or not.
> In chemistry, a pentose is a monosaccharide (simple sugar) with five carbon atoms. The chemical formula of all pentoses is \[{{C}_{5}}{{H}_{10}}{{O}_{5}}\], and their molecular weight is 150.13 g/mol. Like some other monosaccharides, pentoses exist in two forms, open-chain (linear) or closed-chain (cyclic), that easily convert into each other in water solutions. The linear form of a pentose, which usually exists only in solutions, has an open-chain backbone of five carbons. Four of these carbons have one hydroxyl functional group (–OH) each, connected by a single bond, and one has an oxygen atom connected by a double bond (=O), forming a carbonyl group (C=O). The remaining bonds of the carbon atoms are satisfied by six hydrogen atoms. Thus, the structure of the linear form is \[H{{\left( CHOH \right)}_{x}}C\left( =O \right){{\left( CHOH \right)}_{4-x}}H\], where x is 0, 1, or 2.
Since Ribulose satisfies all of these criteria for pentose sugars, therefore the answer to this question is a) True.
Note: Unlike most sugars, ribulose does not exist as a mixture of cyclic forms in equilibrium with its linear form which readily interconvert especially in aqueous solution and only possesses one linear form. The structure of ribulose has two possible enantiomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




