
How many resonating structures of allylic carbocation are present?
A.1
B.2
C.3
D.4

Answer
588k+ views
Hint: A molecule can have resonating structures only when it carries a lone pair or double bond on the atom that is next to the double bond in a molecule. In both the resonance forms of the allylic cation, the positive charge is always located on the terminal carbon atoms and never on the middle carbon.
Complete step by step answer:
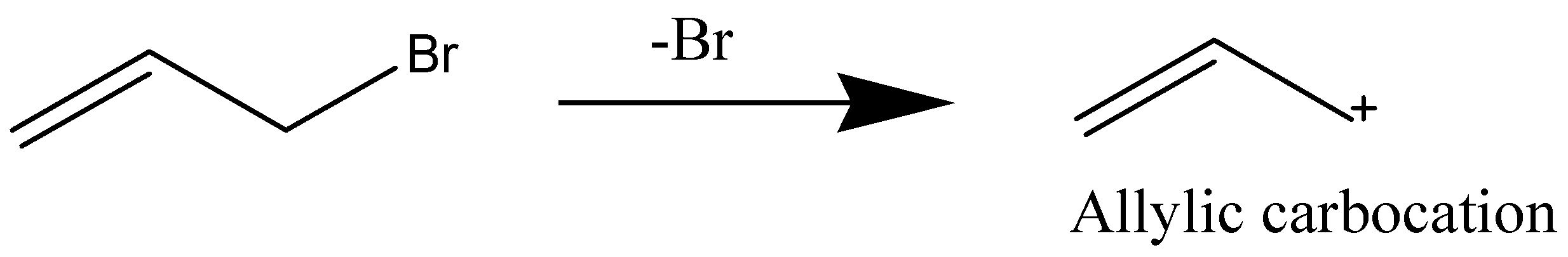
An allylic carbocation is a resonance-stabilized carbocation where in each of the two resonance forms of which the formal charge of +1 is located on an allylic carbon
When more than one correct Lewis structure that satisfies octet rule can be drawn for a molecule, then the structures of that molecule are known as resonating structures.
Resonating structures have two or more possible structures that can be represented by dotted lines or with an arrow between them.
Changing the position of double bond may give you the final product of resonance. And moving lone lone pair, charge and bonds will give you intermediate structures of resonance.
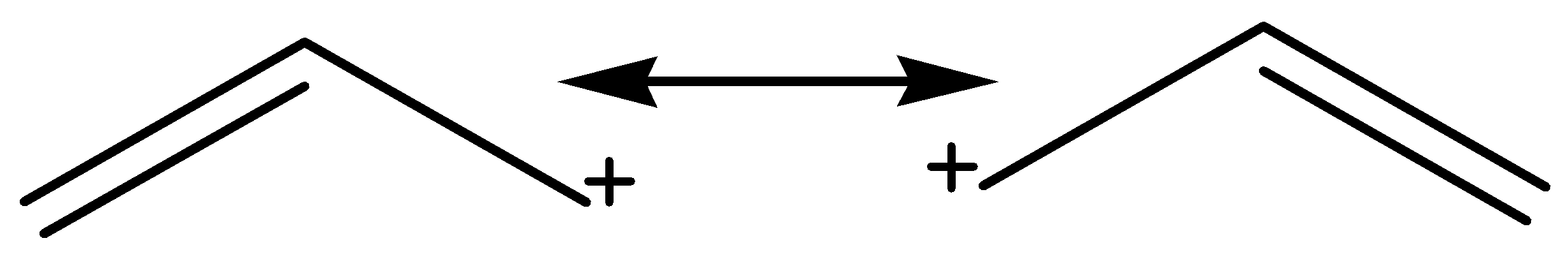
For allylic carbocation, there are two resonance structures observed.
So, following are resonating structures of allylic carbocation:
Hence, the correct option is option B.
Note:
Following points we need to remember while drawing resonating structures:
-Never move atoms in the structure.
-Move only electrons in lone pair and double bond
-Keep framework of bond in molecules intact
-Keep the overall charge of the molecule the same while drawing resonating structure.
-Also we must know that the resonating structures of a molecule represent delocalization of electrons within the molecule itself and so the structures are also known as resonance hybrids.
Complete step by step answer:
An allylic carbocation is a resonance-stabilized carbocation where in each of the two resonance forms of which the formal charge of +1 is located on an allylic carbon
When more than one correct Lewis structure that satisfies octet rule can be drawn for a molecule, then the structures of that molecule are known as resonating structures.
Resonating structures have two or more possible structures that can be represented by dotted lines or with an arrow between them.
Changing the position of double bond may give you the final product of resonance. And moving lone lone pair, charge and bonds will give you intermediate structures of resonance.
For allylic carbocation, there are two resonance structures observed.
So, following are resonating structures of allylic carbocation:

Hence, the correct option is option B.
Note:
Following points we need to remember while drawing resonating structures:
-Never move atoms in the structure.
-Move only electrons in lone pair and double bond
-Keep framework of bond in molecules intact
-Keep the overall charge of the molecule the same while drawing resonating structure.
-Also we must know that the resonating structures of a molecule represent delocalization of electrons within the molecule itself and so the structures are also known as resonance hybrids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




