
Resonance in most of the organic molecules:
A) Increases stability
B) Decreases stability
C) Increases reactivity
D) Increases energy
Answer
555.3k+ views
Hint: We must remember that the resonance is a method of depicting holding in specific particles or particles by the mix of a few contributing structures into a Resonance hybrid in valence bond hypothesis. It has a specific incentive for depicting delocalized electrons inside specific atoms or polyatomic particles where the holding can't be communicated by one single Lewis structure.
Complete step by step answer:
We need to remember that the resonance increases the stability of a molecule.
Now we can discuss the reason:
Resonance fundamentally manages compound holding.
In any case, it is to be noticed that reverberation doesn't bring harmony between the thunderous structures; it is only an approach to speak to various methods of depicting compound bonds.
It is additionally expressed that the more resounding mixtures, the more steady are the structure or particle.
We should take an illustration of reverberation of benzene atoms; it has the accompanying resonant hybrids.
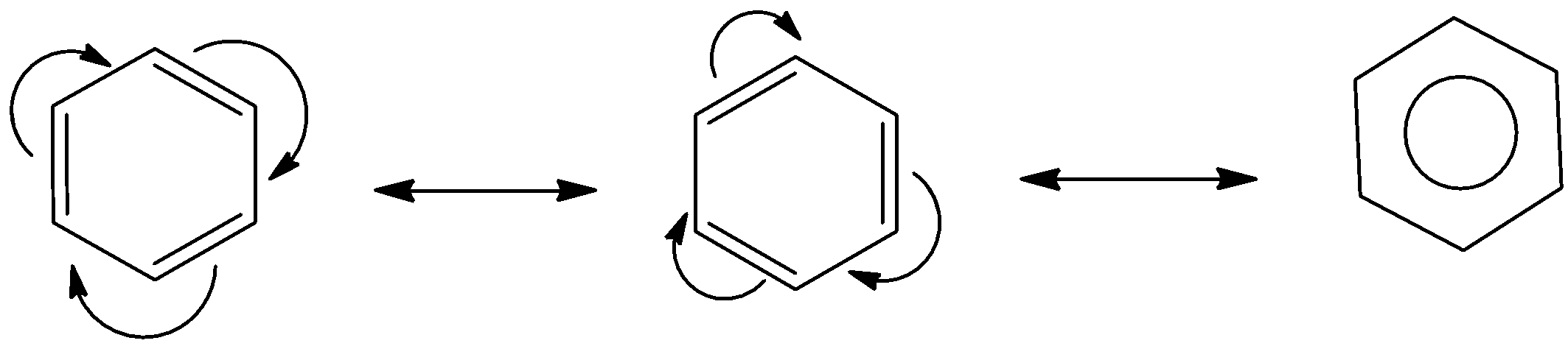
Resonance permits the electron thickness to spread between the iotas or the bonds hence bringing about less electron-electron aversion and in this way brings down the free energy of the half and half, and consequently expands the dependability of the particle.
So, the correct answer is Option A.
Note: We must remember that the resonance is to be recognized from isomerism. Isomers are particles with a similar compound recipe however are unmistakable substance species with various plans of nuclear cores in space. Reverberation benefactors of a particle, then again, can just contrast in the manner in which electrons are officially relegated to iotas in the Lewis structure portrayals of the atom. In particular, when a subatomic structure is supposed to be spoken to by a reverberation cross breed, it doesn't imply that electrons of the particle are "resounding" or moving to and fro between a few arrangements of positions, every one spoken to by a Lewis structure. Or maybe, it implies that the arrangement of contributing structures speaks to a moderate structure, with a solitary, all around characterized calculation and dissemination of electrons.
Complete step by step answer:
We need to remember that the resonance increases the stability of a molecule.
Now we can discuss the reason:
Resonance fundamentally manages compound holding.
In any case, it is to be noticed that reverberation doesn't bring harmony between the thunderous structures; it is only an approach to speak to various methods of depicting compound bonds.
It is additionally expressed that the more resounding mixtures, the more steady are the structure or particle.
We should take an illustration of reverberation of benzene atoms; it has the accompanying resonant hybrids.

Resonance permits the electron thickness to spread between the iotas or the bonds hence bringing about less electron-electron aversion and in this way brings down the free energy of the half and half, and consequently expands the dependability of the particle.
So, the correct answer is Option A.
Note: We must remember that the resonance is to be recognized from isomerism. Isomers are particles with a similar compound recipe however are unmistakable substance species with various plans of nuclear cores in space. Reverberation benefactors of a particle, then again, can just contrast in the manner in which electrons are officially relegated to iotas in the Lewis structure portrayals of the atom. In particular, when a subatomic structure is supposed to be spoken to by a reverberation cross breed, it doesn't imply that electrons of the particle are "resounding" or moving to and fro between a few arrangements of positions, every one spoken to by a Lewis structure. Or maybe, it implies that the arrangement of contributing structures speaks to a moderate structure, with a solitary, all around characterized calculation and dissemination of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




