
What is the relationship between these two compounds?
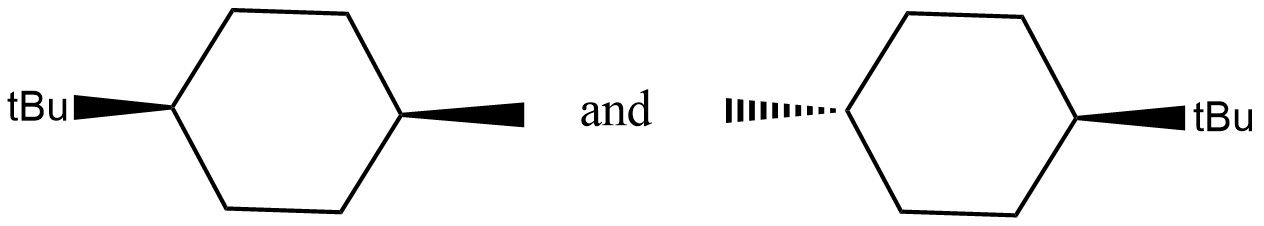
A. Enantiomers
B. Diastereomers
C. Identical
D. None of these
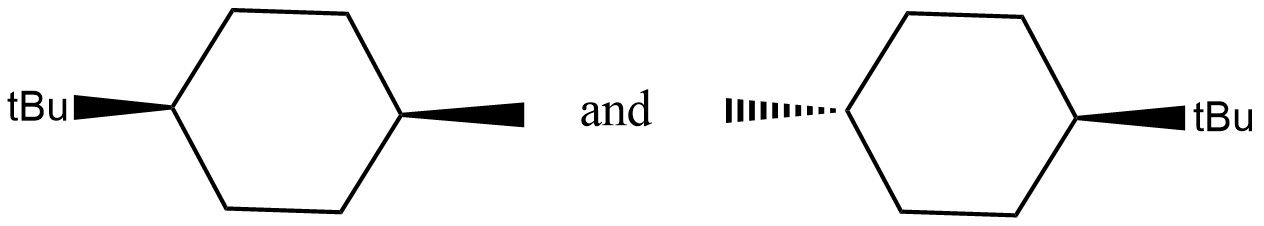
Answer
562.2k+ views
Hint: Isomers are compounds having the same molecular formula but different structures or arrangement of atoms. Stereoisomerism is a special type of isomerism. The types enantiomers, diastereomers, and identical come under this category.
Complete step by step answer:
Stereoisomerism is a special type of isomerism in which the isomers differ in the three dimensional structure. The connectivity of atoms will be the same, but the three dimensional structure will be different.
These two molecules have the same connectivity of atoms. But their three dimensional structure is different. Hence these are stereoisomers. Now let us examine which type of stereoisomers are these compounds. For that first check if they are mirror images to each other. If they are mirror images to each other they may be enantiomers or identical. If the mirror images are superimposable, they will be identical. If they are non-superimposable mirror images, they will be enantiomers. If they are not mirror images, they will be diastereomers. If we place a mirror near any of the given compounds, it doesn’t give the other compound. Hence these are not mirror images. Hence these are diastereomers.
So, the correct answer is Option B.
Note: There is no condition that diastereomers should be optically active. They may or may not be optically active. They show similar but not identical chemical properties.
When two stereoisomers containing one or more stereocenters differ in configuration at one more carbons they are diastereomers. Also they should not be mirror images to each other.
Complete step by step answer:
Stereoisomerism is a special type of isomerism in which the isomers differ in the three dimensional structure. The connectivity of atoms will be the same, but the three dimensional structure will be different.
These two molecules have the same connectivity of atoms. But their three dimensional structure is different. Hence these are stereoisomers. Now let us examine which type of stereoisomers are these compounds. For that first check if they are mirror images to each other. If they are mirror images to each other they may be enantiomers or identical. If the mirror images are superimposable, they will be identical. If they are non-superimposable mirror images, they will be enantiomers. If they are not mirror images, they will be diastereomers. If we place a mirror near any of the given compounds, it doesn’t give the other compound. Hence these are not mirror images. Hence these are diastereomers.
So, the correct answer is Option B.
Note: There is no condition that diastereomers should be optically active. They may or may not be optically active. They show similar but not identical chemical properties.
When two stereoisomers containing one or more stereocenters differ in configuration at one more carbons they are diastereomers. Also they should not be mirror images to each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




