
What is the relationship between the solubility and the temperature? How to explain it to a middle school student (my brother).
Answer
564.6k+ views
Hint: know the basic definition of solubility is that the maximum quantity of solute that we can easily dissolve in certain quantities of solvent at the temperature or pressure which is being specified in the case of gaseous solutes.
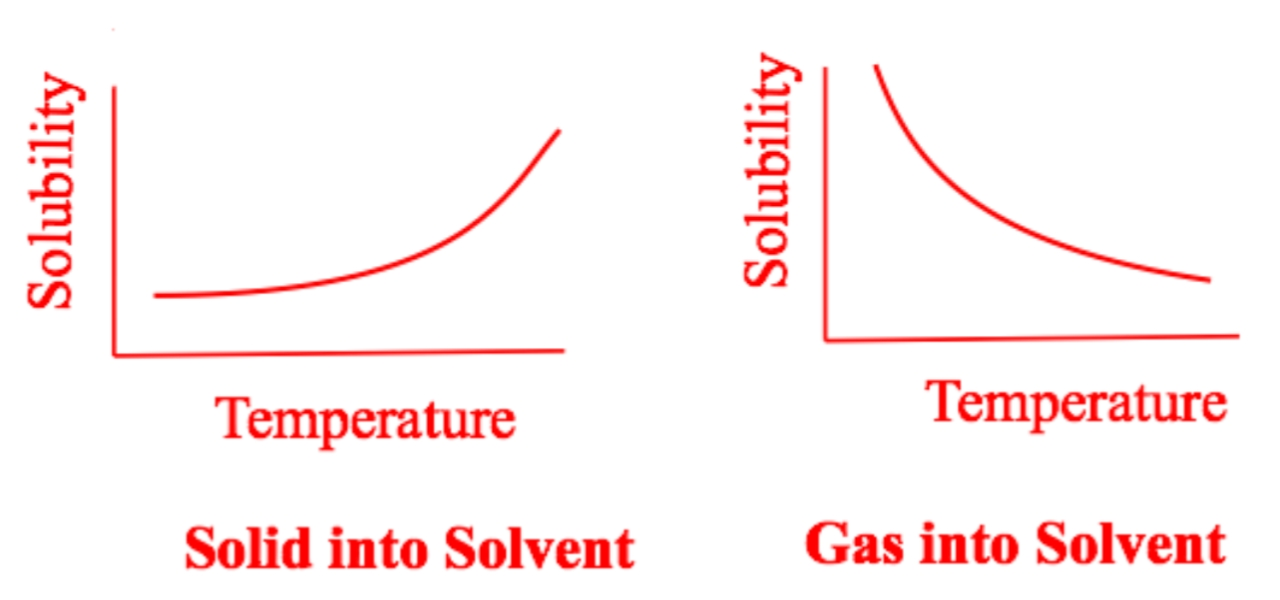
In gaseous state temperature increase, the solubility of a gas decreases as vigorously by the downward trend in the graph.
Complete answer:
The basic Relationship between the solubility and temperature can be given by:
The higher the temperature is, the easier a solid will be able to dissolve.
Likewise the lower is the temperature the harder is for a solid element to dissolve.
This all is because the heat excites the solvent in making it more and more easier for it to break apart or split it away from each other completely.
Meanwhile for in gases
The higher the temperature is, the more there is a decrease in the gas solubility.
The lower the is temperature the higher is a gas solubility in water.
Another property of gas solutes is summarized by Henry law which predicts that the gas becomes more soluble when the pressure above the liquid solution is increased. This property of gas solute can be easily rationalized by using Le Chatelier's principle.
Note: Always Remember that the solubility of most substances depends strongly on the temperature, in case of gas on pressure. The solubility of the most solid and liquidity solute increase with stimuli increasing temperature. The following component of a mixture can often be adequately separated using a fractional crystallization process, which separate compounds ace to their solubility. The solubility of gas decreases with the increased temperature. Henry law describes the relationship between the pressure and the solubility of a gas.
In gaseous state temperature increase, the solubility of a gas decreases as vigorously by the downward trend in the graph.
Complete answer:
The basic Relationship between the solubility and temperature can be given by:
The higher the temperature is, the easier a solid will be able to dissolve.
Likewise the lower is the temperature the harder is for a solid element to dissolve.
This all is because the heat excites the solvent in making it more and more easier for it to break apart or split it away from each other completely.
Meanwhile for in gases
The higher the temperature is, the more there is a decrease in the gas solubility.
The lower the is temperature the higher is a gas solubility in water.

Another property of gas solutes is summarized by Henry law which predicts that the gas becomes more soluble when the pressure above the liquid solution is increased. This property of gas solute can be easily rationalized by using Le Chatelier's principle.
Note: Always Remember that the solubility of most substances depends strongly on the temperature, in case of gas on pressure. The solubility of the most solid and liquidity solute increase with stimuli increasing temperature. The following component of a mixture can often be adequately separated using a fractional crystallization process, which separate compounds ace to their solubility. The solubility of gas decreases with the increased temperature. Henry law describes the relationship between the pressure and the solubility of a gas.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




