
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammonical ${\text{Ni(II)}}$. Which of the following statements is not true?
A) Red complex has a tetrahedral geometry
B) Complex has symmetrical H-bonding
C) Red complex has a square planar geometry
D) Dimethylglyoxime functions as bidentate ligand
Answer
578.4k+ views
Hint: Recall the structure of dimethylglyoxime (DMG). It contains two nitrogen atoms and both can donate its electron density to the metal ion. Also, DMG is a strong field ligand therefore, pairing of electrons will occur in the nickel-DMG complex. After pairing, find the hybridisation of nickel-DMG complex and then its geometry.
Complete step by step solution:
We are given that a red precipitate is obtained when ethanol solution of dimethylglyoxime (DMG) is added to ammonical ${\text{Ni(II)}}$. This chemical reaction can be represented as shown below:
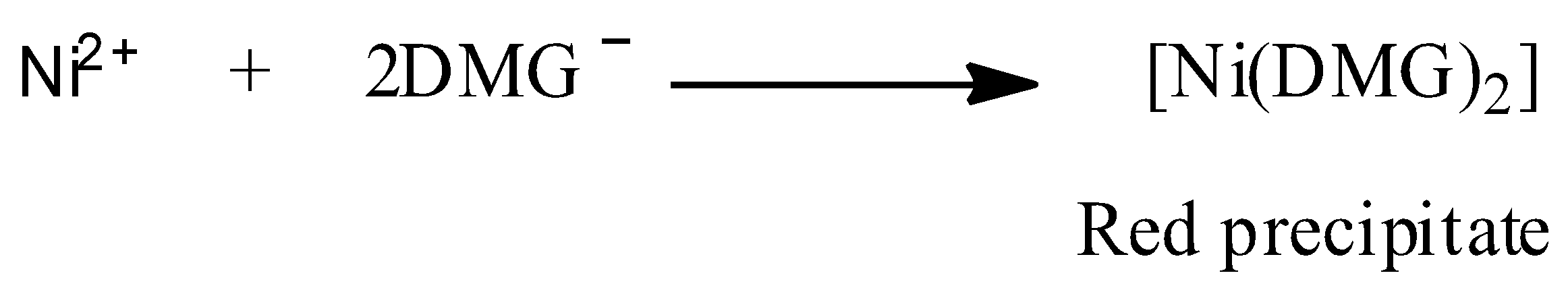
Now, structure of DMG is:
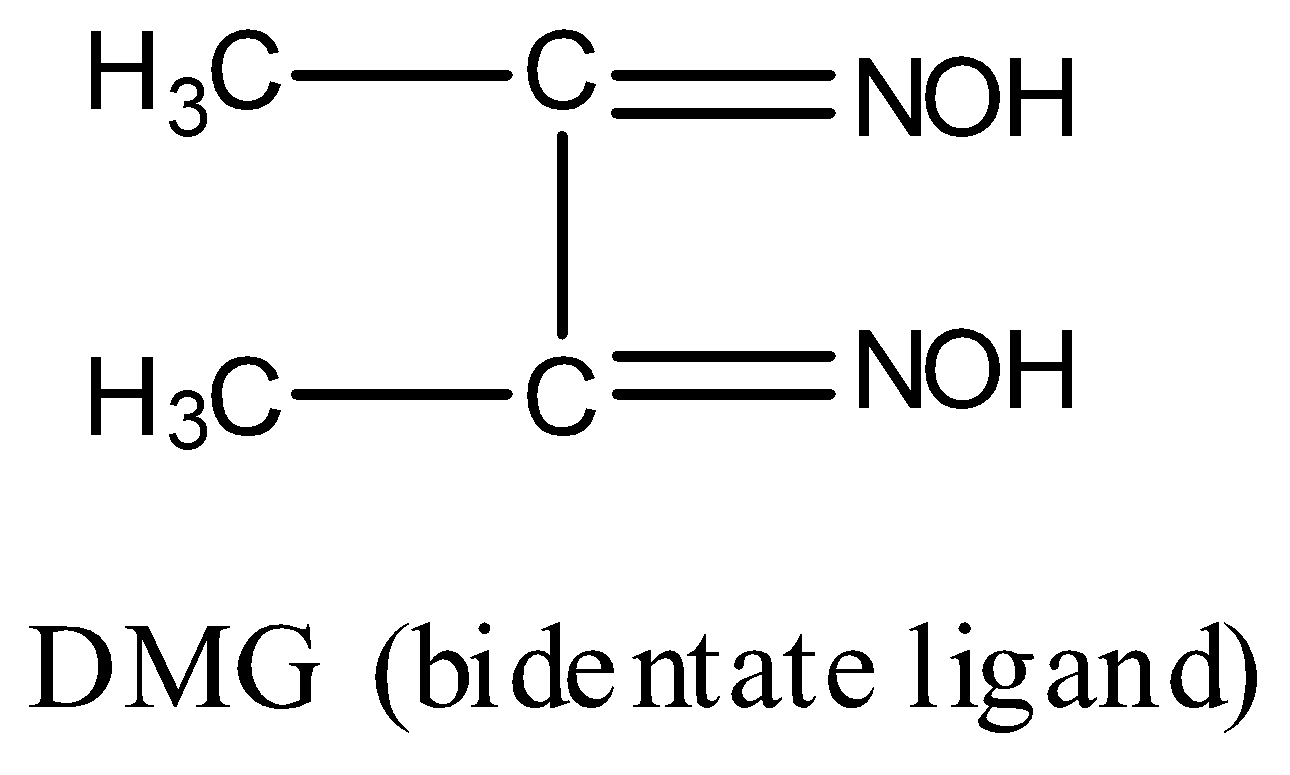
DMG contains two nitrogen atoms and both N will donate electron pairs to the central metal ion, hence, DMG is a bidentate ligand. Thus, the statement given in option D is true.
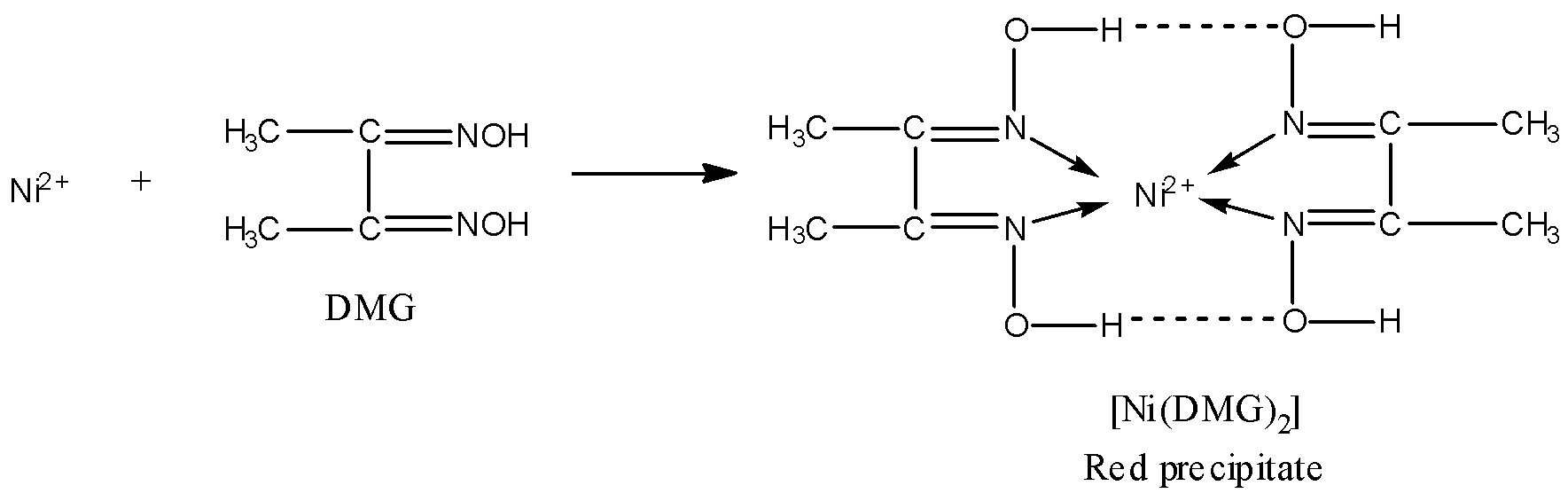
-Now, as you can see in the above nickel-DMG complex that there is symmetrical hydrogen bonding present in the complex. Thus, statement B is also true.
-As you know, Ni is ${d^8}$ system, therefore:
-Valence electronic configuration of $Ni = 3{d^8}4{s^2}$
-Valence electronic configuration of $N{i^{2 + }} = 3{d^8}$
-Now, we know that dimethylglyoxime (DMG) is a strong field ligand, therefore when nickel is present in complex i.e., $[Ni{(DMG)_2}]$, pairing of d-electrons of $N{i^{2 + }}$ will occur. Thus, we can show this as:
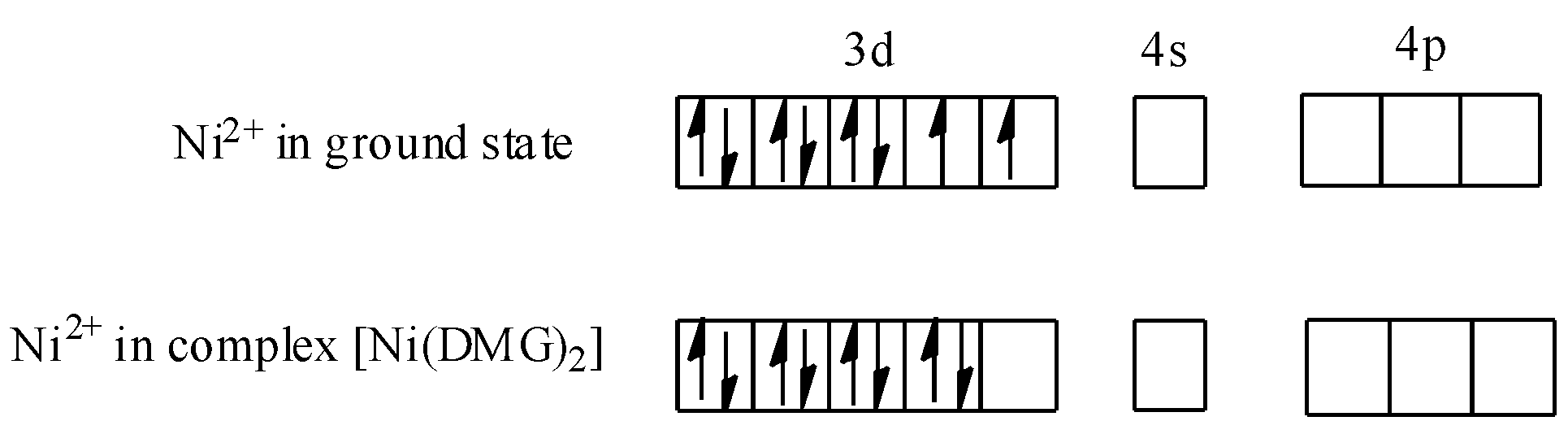
Hence, hybridisation of the $[Ni{(DMG)_2}]$ complex (red complex) will be $ds{p^2}$ and geometry will be square planar. Thus, statement C is also true. On concluding, we find that the statement given in option A i.e., red complex has a tetrahedral geometry, is not true.
Thus, option A is the answer.
Note: When coordination number of a complex is 4 i.e., four ligands are linked to central metal ions, then the geometry will be either tetrahedral or square planar. For tetrahedral geometry, hybridisation is $s{p^3}$ whereas for square planar geometry, hybridisation is $ds{p^2}$.
Complete step by step solution:
We are given that a red precipitate is obtained when ethanol solution of dimethylglyoxime (DMG) is added to ammonical ${\text{Ni(II)}}$. This chemical reaction can be represented as shown below:
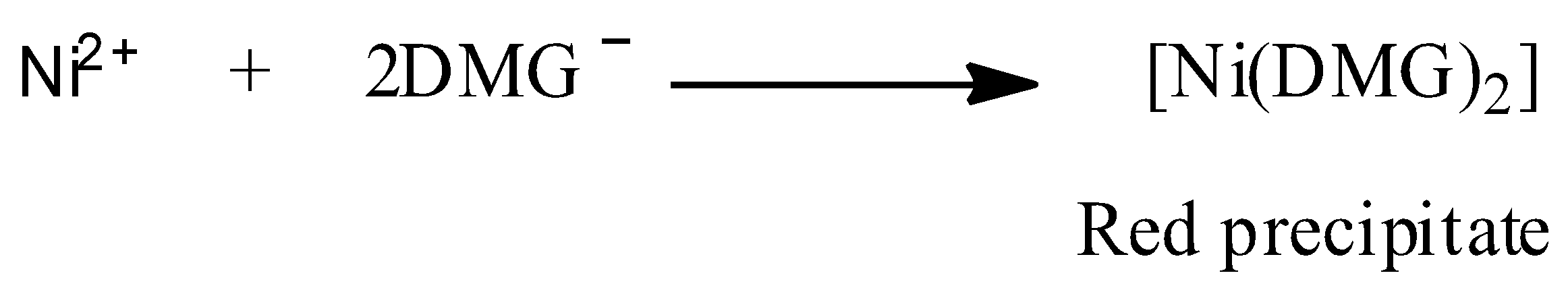
Now, structure of DMG is:

DMG contains two nitrogen atoms and both N will donate electron pairs to the central metal ion, hence, DMG is a bidentate ligand. Thus, the statement given in option D is true.

-Now, as you can see in the above nickel-DMG complex that there is symmetrical hydrogen bonding present in the complex. Thus, statement B is also true.
-As you know, Ni is ${d^8}$ system, therefore:
-Valence electronic configuration of $Ni = 3{d^8}4{s^2}$
-Valence electronic configuration of $N{i^{2 + }} = 3{d^8}$
-Now, we know that dimethylglyoxime (DMG) is a strong field ligand, therefore when nickel is present in complex i.e., $[Ni{(DMG)_2}]$, pairing of d-electrons of $N{i^{2 + }}$ will occur. Thus, we can show this as:

Hence, hybridisation of the $[Ni{(DMG)_2}]$ complex (red complex) will be $ds{p^2}$ and geometry will be square planar. Thus, statement C is also true. On concluding, we find that the statement given in option A i.e., red complex has a tetrahedral geometry, is not true.
Thus, option A is the answer.
Note: When coordination number of a complex is 4 i.e., four ligands are linked to central metal ions, then the geometry will be either tetrahedral or square planar. For tetrahedral geometry, hybridisation is $s{p^3}$ whereas for square planar geometry, hybridisation is $ds{p^2}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




