
What reagent is used in the Hinsberg test of amines?
(a) $({ CH }_{ 3 }CO{ ) }_{ 2 }O$ and pyridine
(b) ${ C }_{ 6 }{ H }_{ 5 }{ SO }_{ 2 }Cl$ in aq. NaOH
(c) $Na{ NO }_{ 2 }$ in aq. $Na{ NO }_{ 2 }$
(d) ${ CH }_{ 3 }$ (excess) followed by AgOH
Answer
585k+ views
Hint: In order to distinguish between the primary, secondary and the tertiary amines we use Hinsberg’s reagent. Hinsberg’s reagent is prepared by the chlorination of benzene sulphonic acid by using phosphorus oxychloride. Any other suitable chlorinating agent can also be used.
Complete step by step solution:
Hinsberg’s reagent is used in order to distinguish between primary, secondary and tertiary amines. Let us first understand these three types of amines:
In primary amines or ${ 1 }^{ o }$ amines two ${ sp }^{ 3 }$ hybrid orbitals of Nitrogen overlap with the 1s orbital of Hydrogen atoms, one ${ sp }^{ 3 }$ orbital contains a lone pair while the remaining ${ sp }^{ 3 }$ orbital overlaps with the ${ sp }^{ 3 }$ orbital of a carbon atom belonging to an alkyl group or with a ${ sp }^{ 2 }$ orbital of a carbon atom belonging to an aryl group.
In secondary or ${ 2 }^{ o }$ amines one ${ sp }^{ 3 }$ hybrid orbital of Nitrogen overlaps with the 1s orbital of a Hydrogen atom, one ${ sp }^{ 3 }$ orbital contains a lone pair while the remaining two ${ sp }^{ 3 }$ orbitals overlap with the ${ sp }^{ 3 }$ orbitals of carbon atoms of two alkyl groups or with ${ sp }^{ 2 }$ orbitals of carbon atoms of two aryl groups.
In tertiary or ${ 3 }^{ o }$ amines one ${ sp }^{ 3 }$ orbital of Nitrogen atom contains a lone pair while the remaining three ${ sp }^{ 3 }$ orbitals overlap with the ${ sp }^{ 3 }$ orbitals of carbon atoms of three alkyl groups or with ${ sp }^{ 2 }$ orbitals of carbon atoms of three aryl groups.
In order to distinguish between them, Hinsberg’s reagent (Benzenesulfonyl chloride) is used.
A primary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give a clear solution. When this solution is acidified, an insoluble N-alkyl benzene sulphonamide is produced. The reaction is given below:
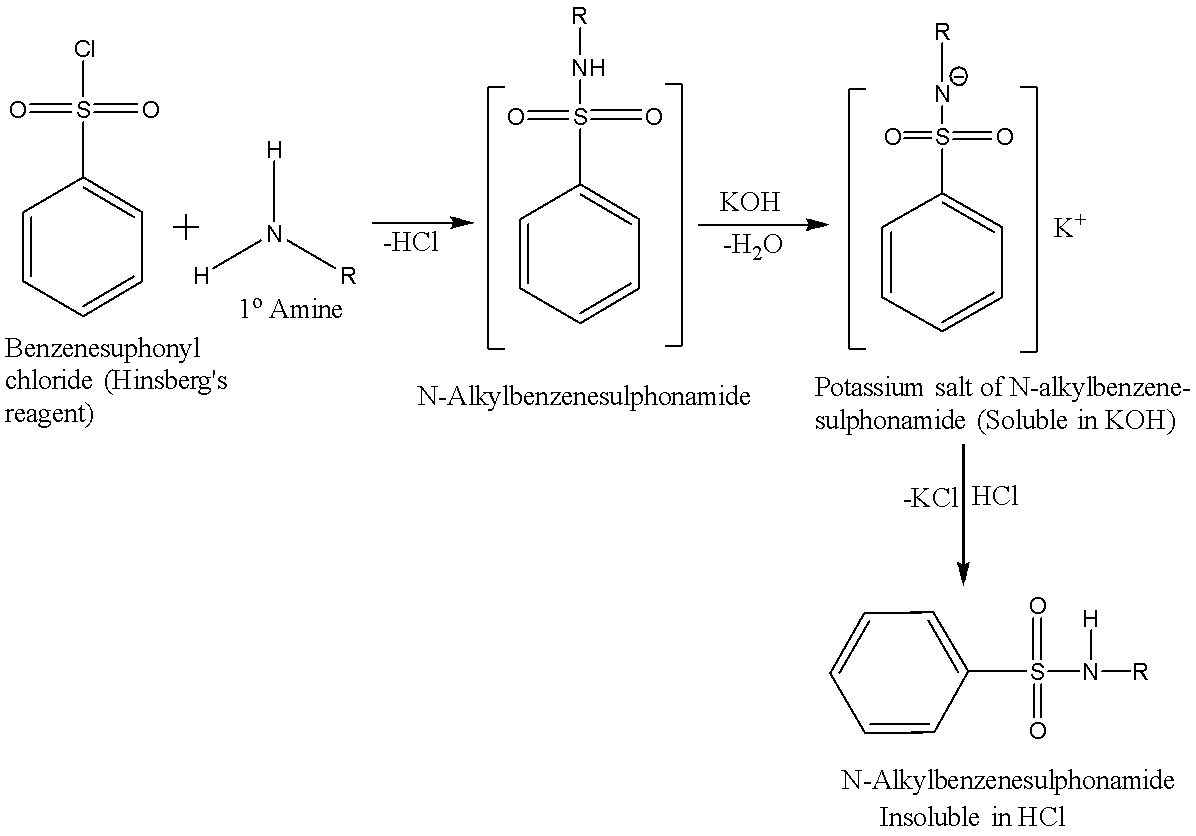
A secondary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give an insoluble N,N-dialkyl benzenesulfonamide and there is no change observed when acid is added to it. The reaction is given below:
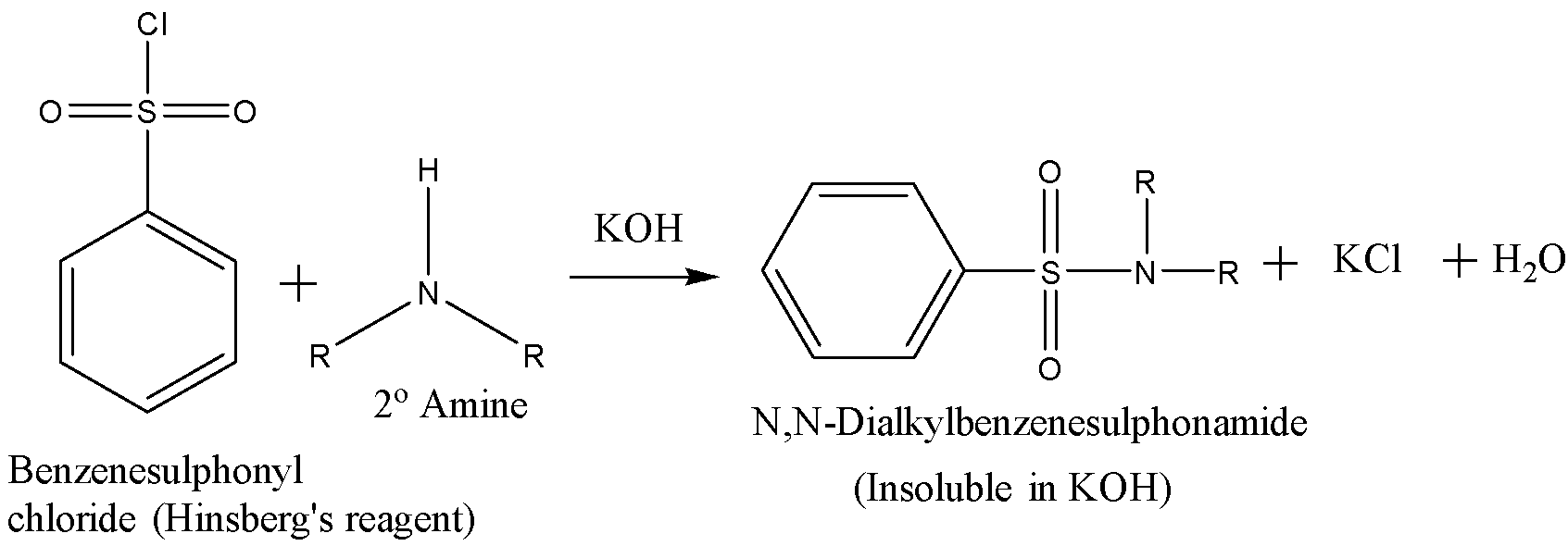
A tertiary amine does not react with Hinsberg’s reagent and remains insoluble in the alkaline solution. On acidification of the solution a clear solution is obtained because of the formation of the ammonium salt. The reaction is given below:
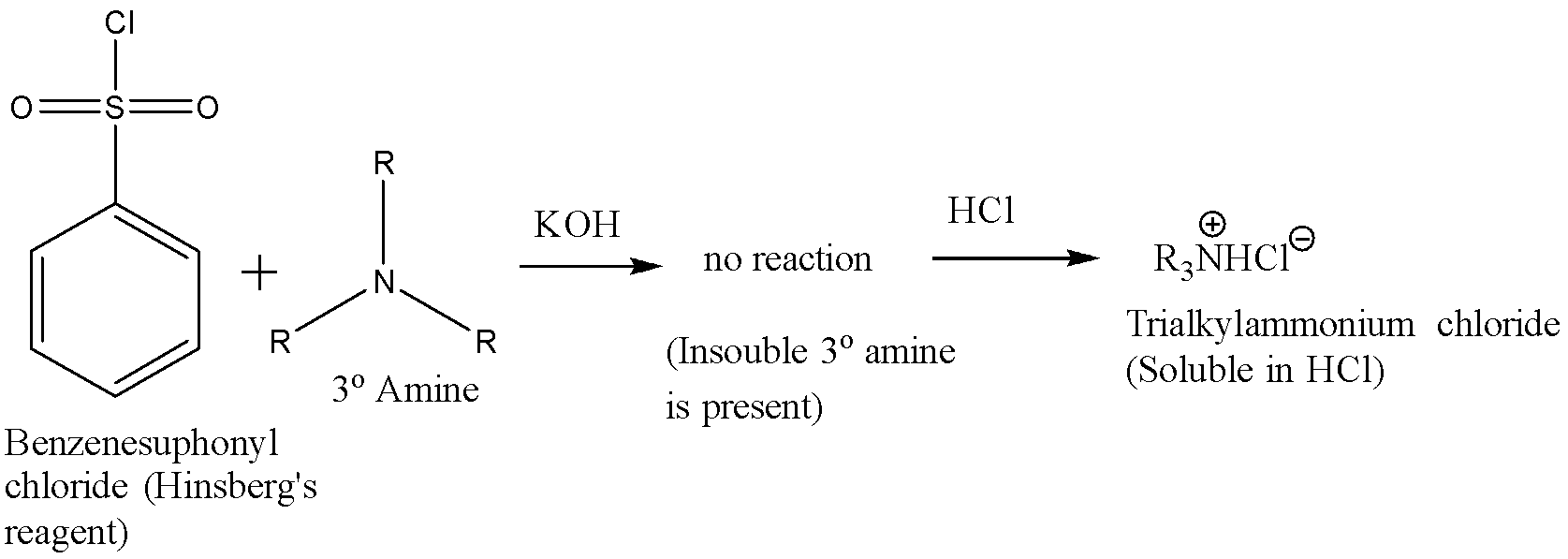
Hence the correct answer is (b) ${ C }_{ 6 }{ H }_{ 5 }{ SO }_{ 2 }Cl$ in aq. NaOH.
Note: For providing the alkaline medium, both NaOH and KOH can be used with Hinsberg’s reagent. A primary amine forms a potassium salt of N-Alkyl Benzene Sulphonamide when it reacts with the Hinsberg’s reagent in the presence of aq. KOH since a primary amine has an acidic hydrogen on the N-atom of the amine group.
Complete step by step solution:
Hinsberg’s reagent is used in order to distinguish between primary, secondary and tertiary amines. Let us first understand these three types of amines:
In primary amines or ${ 1 }^{ o }$ amines two ${ sp }^{ 3 }$ hybrid orbitals of Nitrogen overlap with the 1s orbital of Hydrogen atoms, one ${ sp }^{ 3 }$ orbital contains a lone pair while the remaining ${ sp }^{ 3 }$ orbital overlaps with the ${ sp }^{ 3 }$ orbital of a carbon atom belonging to an alkyl group or with a ${ sp }^{ 2 }$ orbital of a carbon atom belonging to an aryl group.
In secondary or ${ 2 }^{ o }$ amines one ${ sp }^{ 3 }$ hybrid orbital of Nitrogen overlaps with the 1s orbital of a Hydrogen atom, one ${ sp }^{ 3 }$ orbital contains a lone pair while the remaining two ${ sp }^{ 3 }$ orbitals overlap with the ${ sp }^{ 3 }$ orbitals of carbon atoms of two alkyl groups or with ${ sp }^{ 2 }$ orbitals of carbon atoms of two aryl groups.
In tertiary or ${ 3 }^{ o }$ amines one ${ sp }^{ 3 }$ orbital of Nitrogen atom contains a lone pair while the remaining three ${ sp }^{ 3 }$ orbitals overlap with the ${ sp }^{ 3 }$ orbitals of carbon atoms of three alkyl groups or with ${ sp }^{ 2 }$ orbitals of carbon atoms of three aryl groups.
In order to distinguish between them, Hinsberg’s reagent (Benzenesulfonyl chloride) is used.
A primary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give a clear solution. When this solution is acidified, an insoluble N-alkyl benzene sulphonamide is produced. The reaction is given below:

A secondary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give an insoluble N,N-dialkyl benzenesulfonamide and there is no change observed when acid is added to it. The reaction is given below:

A tertiary amine does not react with Hinsberg’s reagent and remains insoluble in the alkaline solution. On acidification of the solution a clear solution is obtained because of the formation of the ammonium salt. The reaction is given below:

Hence the correct answer is (b) ${ C }_{ 6 }{ H }_{ 5 }{ SO }_{ 2 }Cl$ in aq. NaOH.
Note: For providing the alkaline medium, both NaOH and KOH can be used with Hinsberg’s reagent. A primary amine forms a potassium salt of N-Alkyl Benzene Sulphonamide when it reacts with the Hinsberg’s reagent in the presence of aq. KOH since a primary amine has an acidic hydrogen on the N-atom of the amine group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




