
Reactivity order of ${{S}_{N}}1$ reaction for the following compounds is
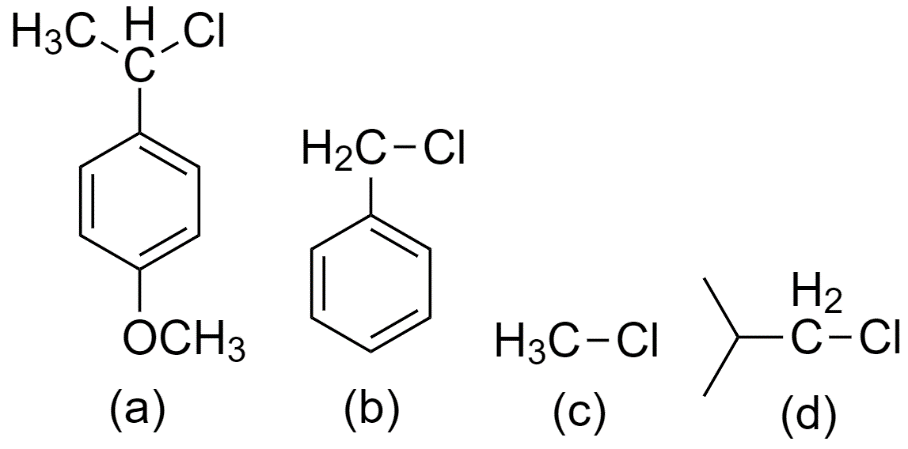
(A) a > b > c > d
(B) a > d > c > b
(C) c > d > b > a
(D) a > b > d > c

Answer
532.2k+ views
Hint: The stability of a compound in a ${{S}_{N}}1$ reaction is dependent on the stability of the carbocation formed by the compound. When the stability of a carbocation intermediate is increased, the activation energy of the reaction is lowered, and hence the speed of the reaction increases.
Complete step-by-step solution:
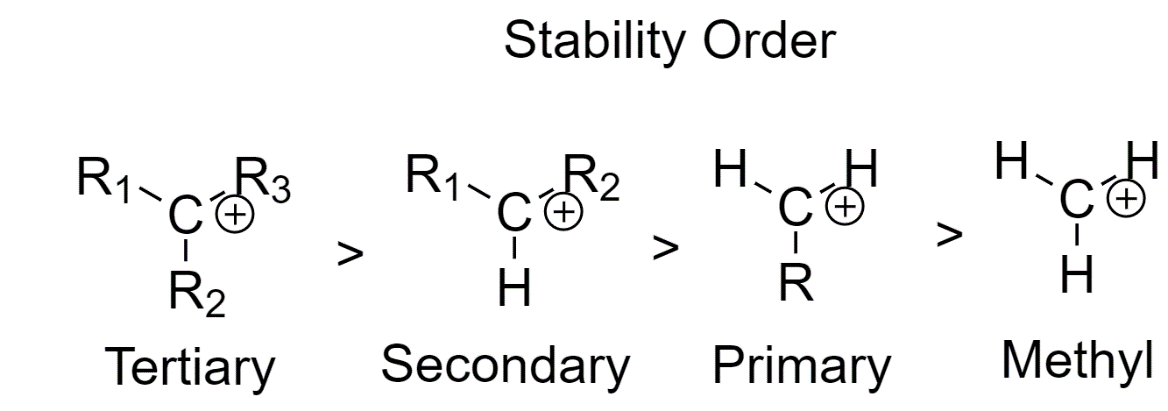
The stability of the carbocation is mainly dependent on three factors
1. The neighboring carbon atoms determine the stability of the carbocation
As we go from primary carbons to secondary carbons and finally to tertiary carbons, the stability of carbocation increases.
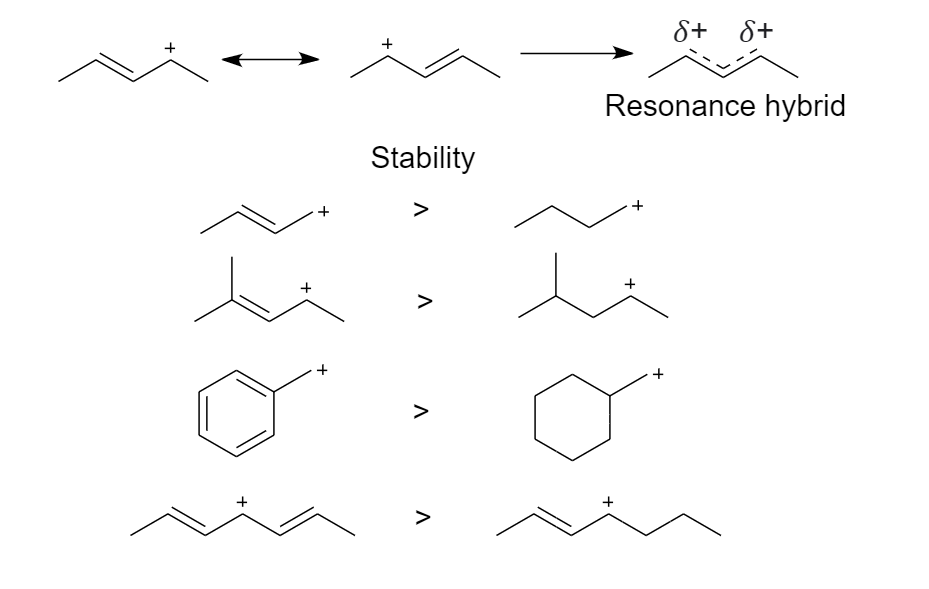
2. The neighboring carbon-carbon multiple bonds stabilize the carbocation
Delocalization occurs when the positive charge is shared between multiple atoms. This happens when the p-orbitals of the $\pi $ bond overlap with the p-orbitals of the carbocation.
Delocalization (resonance) stabilizes a carbocation such that even the most unstable primary carbocation will participate in nucleophilic substitution reactions. It is an additive effect.
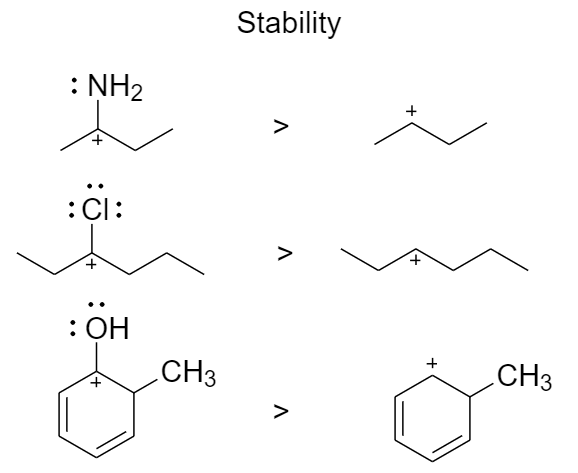
3. Adjacent atoms bearing lone pairs stabilize carbocations
When a neighboring atom donates a pair of electrons, $\pi $ donors, there is invariably a formation of a double bond and hence there is an increase in stability of the carbocation.
The basicity of the donor atom affects the stability and hence oxygen and nitrogen are the most powerful $\pi $ donors.
Now, the compounds (a), (b), (c), and (d) form the following carbocations
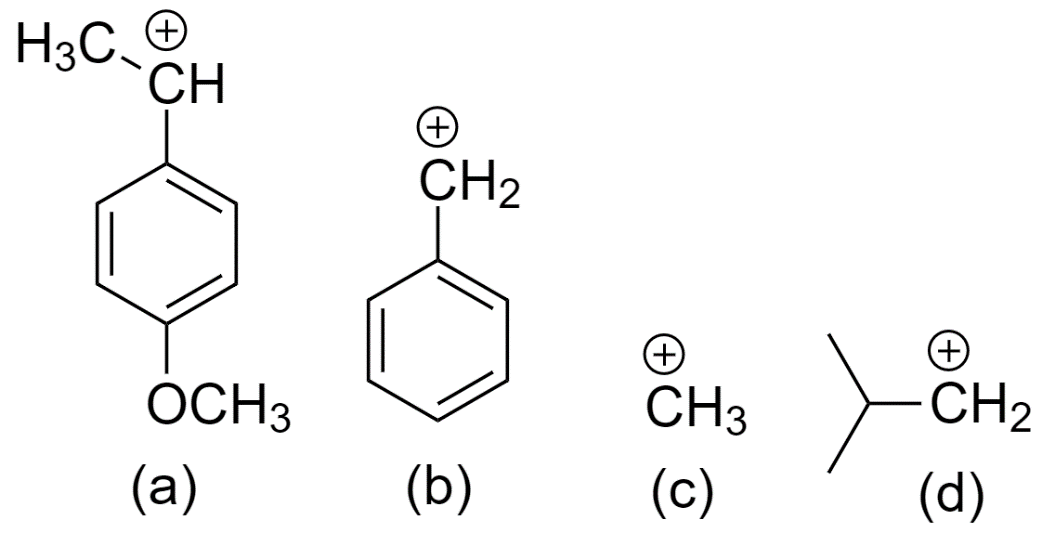
From the above three factors, we can determine that the most stable carbocation is formed by (a) since it is a secondary carbocation and is attached to a benzene ring.
Followed by carbocation formed by (b) since it is attached to the benzene ring hence increases the resonance effect.
Followed by the carbocation formed by (d) since it is a primary carbocation.
And the least stable carbocation is formed by (c).
So, the reactivity order of ${{S}_{N}}1$ reaction for the given compounds is option (D) a > b > d > c.
Note:
A molecule in which the carbon atom forms three bonds and has a positive charge is known as a carbocation. The simplest example of a carbocation is metheniun or methyl carbocation.
The presence of more than one positive charge on a carbon atom is rare.
Since carbocation has a positive charge, it only has six electrons in its valence shell and hence is deficient in electrons. It is unstable and is usually formed as an intermediate in substitution reactions, elimination reactions, electrophilic aromatic substitution reactions, etc.
Complete step-by-step solution:
The stability of the carbocation is mainly dependent on three factors
1. The neighboring carbon atoms determine the stability of the carbocation
As we go from primary carbons to secondary carbons and finally to tertiary carbons, the stability of carbocation increases.

2. The neighboring carbon-carbon multiple bonds stabilize the carbocation
Delocalization occurs when the positive charge is shared between multiple atoms. This happens when the p-orbitals of the $\pi $ bond overlap with the p-orbitals of the carbocation.
Delocalization (resonance) stabilizes a carbocation such that even the most unstable primary carbocation will participate in nucleophilic substitution reactions. It is an additive effect.

3. Adjacent atoms bearing lone pairs stabilize carbocations
When a neighboring atom donates a pair of electrons, $\pi $ donors, there is invariably a formation of a double bond and hence there is an increase in stability of the carbocation.
The basicity of the donor atom affects the stability and hence oxygen and nitrogen are the most powerful $\pi $ donors.

Now, the compounds (a), (b), (c), and (d) form the following carbocations

From the above three factors, we can determine that the most stable carbocation is formed by (a) since it is a secondary carbocation and is attached to a benzene ring.
Followed by carbocation formed by (b) since it is attached to the benzene ring hence increases the resonance effect.
Followed by the carbocation formed by (d) since it is a primary carbocation.
And the least stable carbocation is formed by (c).
So, the reactivity order of ${{S}_{N}}1$ reaction for the given compounds is option (D) a > b > d > c.
Note:
A molecule in which the carbon atom forms three bonds and has a positive charge is known as a carbocation. The simplest example of a carbocation is metheniun or methyl carbocation.
The presence of more than one positive charge on a carbon atom is rare.
Since carbocation has a positive charge, it only has six electrons in its valence shell and hence is deficient in electrons. It is unstable and is usually formed as an intermediate in substitution reactions, elimination reactions, electrophilic aromatic substitution reactions, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




