
Reactivity of \[HCHO\] with the given Grignard reagent in the decreasing order:
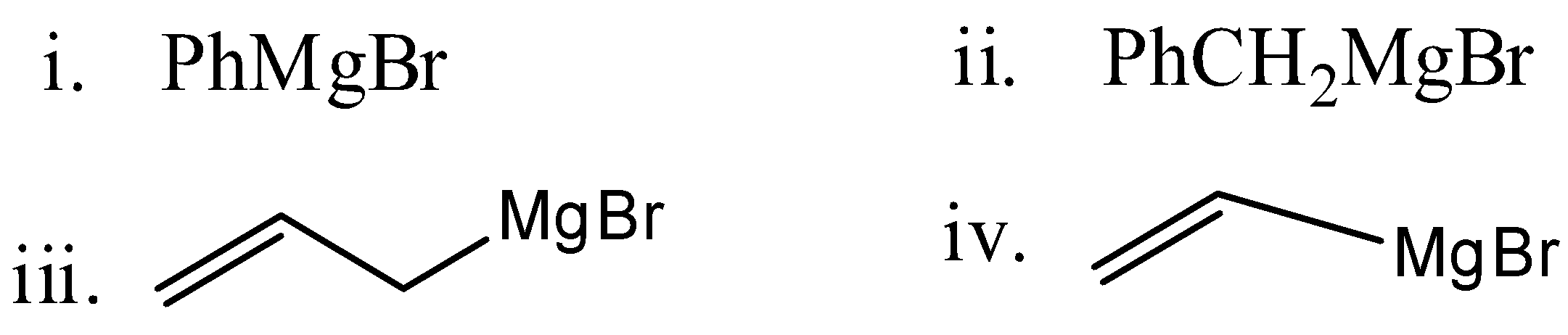
a.) (iv) ˃ (iii) ˃ (ii) ˃ (i)
b.) (i) ˃ (ii) ˃ (iii) ˃ (iv)
c.) (iii) ˃ (ii) ˃ (i) ˃ (iv)
d.) (ii) ˃ (iii) ˃ (i) ˃ (iv)
Answer
584.7k+ views
Hint: Alkyl or aryl magnesium halide is known as Grignard reagent (RMgX). We can synthesize Grignard reagents by the reaction of magnesium metal with alkyl or alkenyl halides. Grignard reagent acts as a good nucleophile, reacts with electron deficient carbons like carbonyl compounds and etc.
Complete step by step answer:
Here we are supposed to find the reactivity of formaldehyde towards the given Grignard reagents.
We know that in Grignard reagent, the hydrocarbon part acts as a nucleophile from its starting material.
i. \[PhMgBr\xrightarrow[{}]{}P{{h}^{-}}\]
The benzyl magnesium bromide gives the following nucleophile from its starting material.
ii. \[PhC{{H}_{2}}MgBr\xrightarrow{{}}PhC{{H}_{2}}^{-}\]
The allyl magnesium bromide gives the following nucleophile from its starting material.
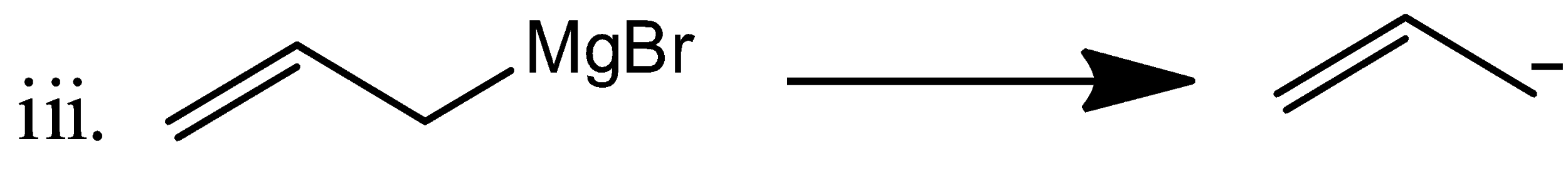
The vinyl magnesium bromide gives the following nucleophile from its starting material.
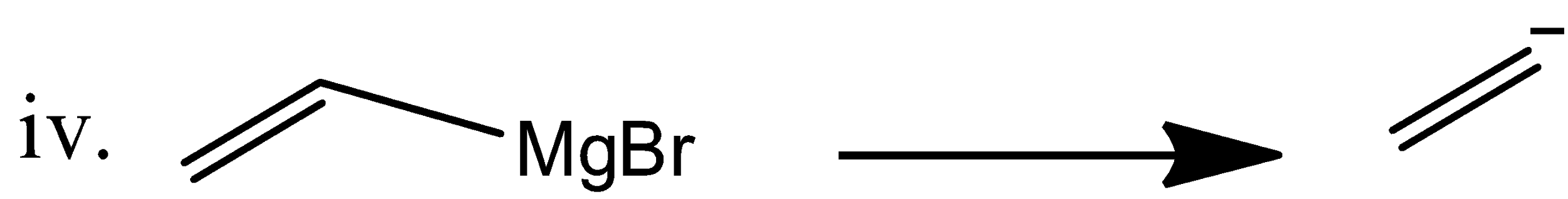
Now among the four Grignard reagents the benzyl anion (ii) is more nucleophilic towards formaldehyde due to its stability and resonance.
After that allyl anion (iii) is more nucleophilic towards formaldehyde due to its stability and resonance.
After that phenyl anion (i) is more nucleophilic towards formaldehyde.
After that vinyl anion (iv) is more nucleophilic towards formaldehyde.
Therefore the decreasing reactivity order of Grignard reagent towards formaldehyde is as follows.
(ii) ˃ (iii) ˃ (i) ˃ (iv)
So, the correct answer is “Option D”.
Note: In the Grignard reagent the hydrocarbon part will have a negative charge and MgBr gets positive charge. Due to the negative charge on the hydrocarbon part it acts as a good nucleophile.
Complete step by step answer:
Here we are supposed to find the reactivity of formaldehyde towards the given Grignard reagents.
We know that in Grignard reagent, the hydrocarbon part acts as a nucleophile from its starting material.
i. \[PhMgBr\xrightarrow[{}]{}P{{h}^{-}}\]
The benzyl magnesium bromide gives the following nucleophile from its starting material.
ii. \[PhC{{H}_{2}}MgBr\xrightarrow{{}}PhC{{H}_{2}}^{-}\]
The allyl magnesium bromide gives the following nucleophile from its starting material.
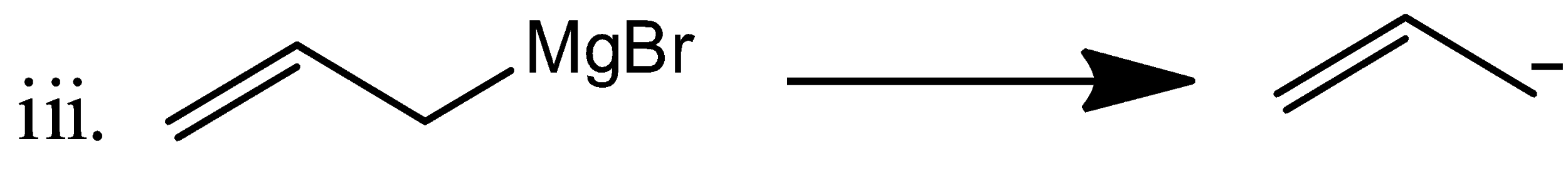
The vinyl magnesium bromide gives the following nucleophile from its starting material.
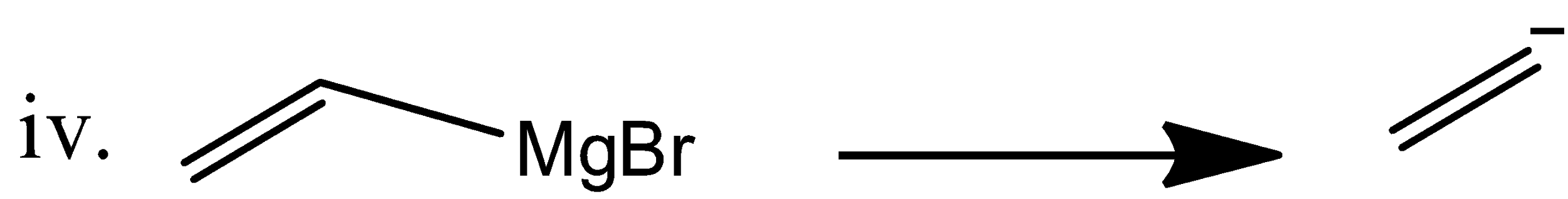
Now among the four Grignard reagents the benzyl anion (ii) is more nucleophilic towards formaldehyde due to its stability and resonance.
After that allyl anion (iii) is more nucleophilic towards formaldehyde due to its stability and resonance.
After that phenyl anion (i) is more nucleophilic towards formaldehyde.
After that vinyl anion (iv) is more nucleophilic towards formaldehyde.
Therefore the decreasing reactivity order of Grignard reagent towards formaldehyde is as follows.
(ii) ˃ (iii) ˃ (i) ˃ (iv)
So, the correct answer is “Option D”.
Note: In the Grignard reagent the hydrocarbon part will have a negative charge and MgBr gets positive charge. Due to the negative charge on the hydrocarbon part it acts as a good nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




