
Reaction of bromine water phenol gives white ppt of:
A. o – bromophenol
B. p – bromophenol
C. 2,4,6 – tribromophenol
D. m – bromophenol
Answer
605.4k+ views
Hint: When bromine water is added to a solution of phenol in water, the bromine water is decolourised and a white precipitate is formed which smells of antiseptic.
Complete step-by-step answer:
We know that the reaction of phenol and water with bromine is known as bromination of phenol. Solvent has a great influence on the reaction. In different solvents, different products are obtained.
Bromination of phenol is a substitution reaction. Where the bromine replaces hydrogen present in the benzene ring of phenol. In the water solvent when phenol treated with \[B{{r}_{2}}\] gives a polybromo derivative in which all hydrogen atoms at ortho, meta, and para positions with respect to the \[-OH\] group are replaced by bromine atoms. It is so because in aqueous medium phenol ionizes to form peroxide ion. Due to the presence of negative ions the ring gets highly activated and tri substitution occurs and the formation of 2,4,6 – tribromophenol takes place.
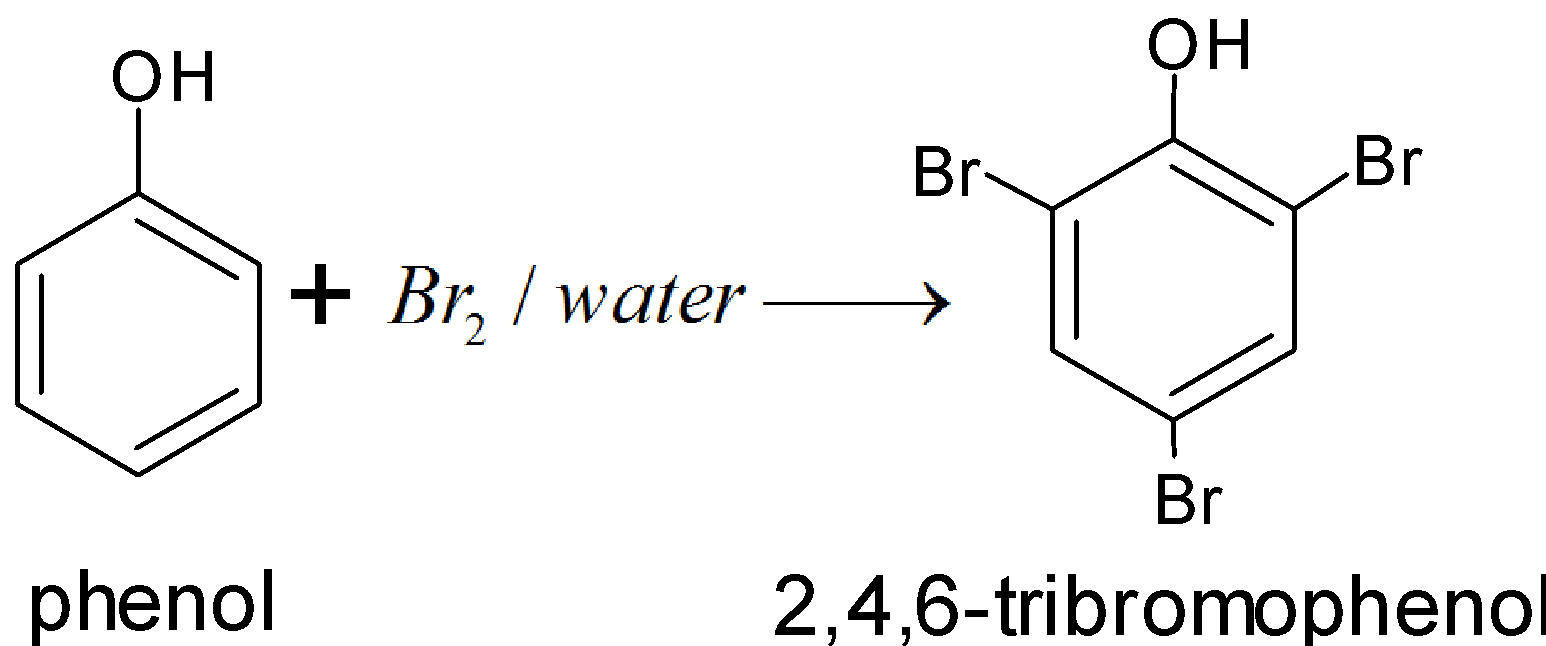
So, the correct answer is “D”.
A, B, C are incorrect because these product formation takes place in non-polar solvents.
Note: In non-polar solvents the ionization of phenol is greatly suppressed. As a result, \[-OH\] group donates electrons to benzene rings to a small extent only which results in slight activation of ring and mono-substitution occurs.
Complete step-by-step answer:
We know that the reaction of phenol and water with bromine is known as bromination of phenol. Solvent has a great influence on the reaction. In different solvents, different products are obtained.
Bromination of phenol is a substitution reaction. Where the bromine replaces hydrogen present in the benzene ring of phenol. In the water solvent when phenol treated with \[B{{r}_{2}}\] gives a polybromo derivative in which all hydrogen atoms at ortho, meta, and para positions with respect to the \[-OH\] group are replaced by bromine atoms. It is so because in aqueous medium phenol ionizes to form peroxide ion. Due to the presence of negative ions the ring gets highly activated and tri substitution occurs and the formation of 2,4,6 – tribromophenol takes place.
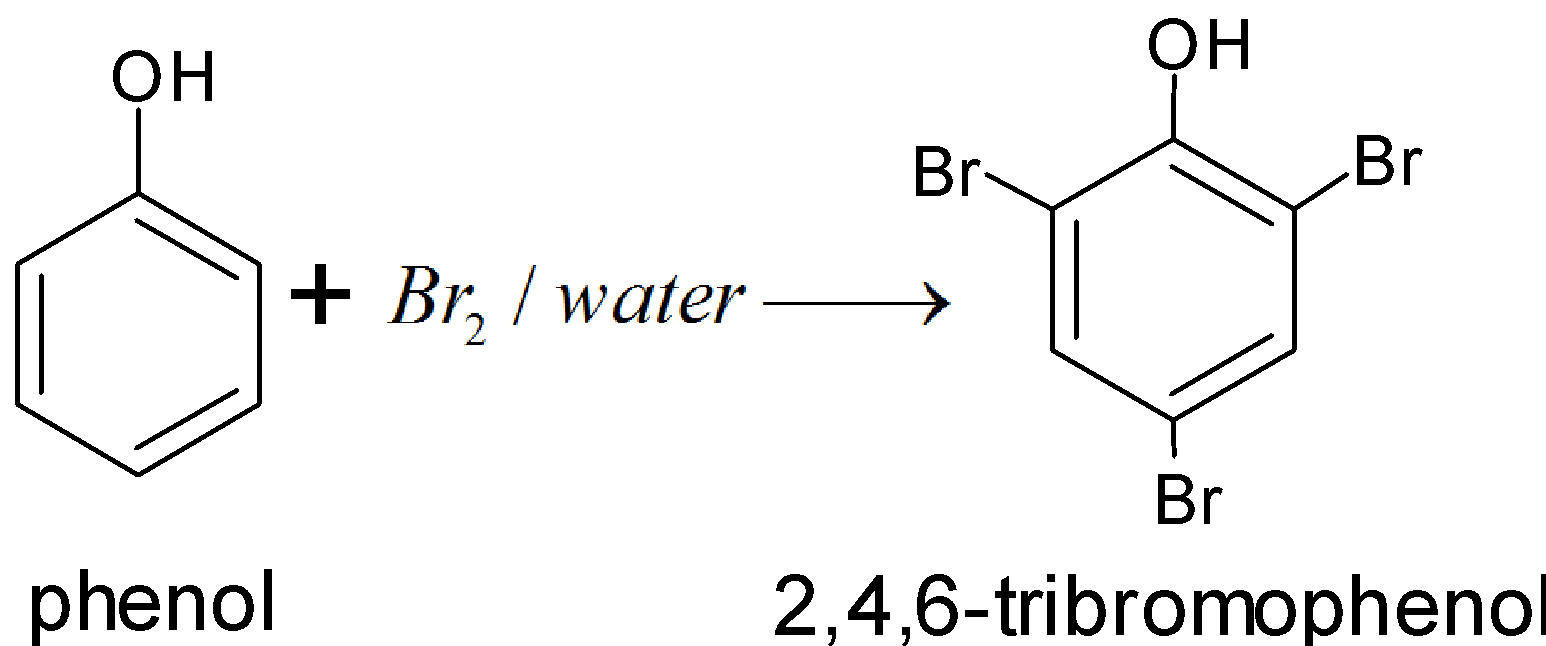
So, the correct answer is “D”.
A, B, C are incorrect because these product formation takes place in non-polar solvents.
Note: In non-polar solvents the ionization of phenol is greatly suppressed. As a result, \[-OH\] group donates electrons to benzene rings to a small extent only which results in slight activation of ring and mono-substitution occurs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




