
Radial symmetry is seen in flowers of
(a)Cassia
(b)Brassica
(c)Trifolium
(d)Pisum
Answer
594.9k+ views
Hint: When the different parts of each whorl are similar, the flower is regular and is called an actinomorphic flower. This means that all members of a whorl are the same in size and shape.
Complete answer:
Brassica has Actinomorphic flowers i.e it is a flower with radial symmetry. All the petals of brassica are similar in size and shape. The flower can be cut into two equal halves from any plane.
Additional information:
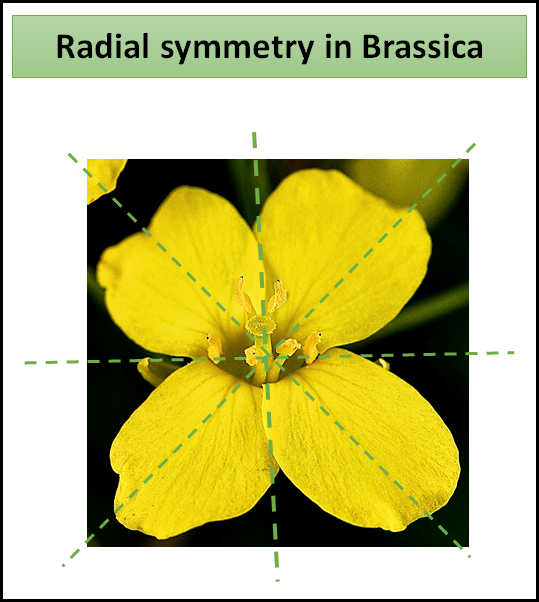
-A flower is supposed to be symmetrical when each whorl comprises an equal number of members or when the members of any one whorl are multiples of that before it.
-Thus, a symmetrical flower may have three sepals, three petals, three stamens, and three carpels, or the quantity of any of these members may be a multiple of three.
-A flower is dimerous in which the parts are in twos; trimerous, tetramerous, or pentamerous, in threes, fours, or fives, respectively.
-When the different parts of each whorl are identical, the flower is regular and is called as an actinomorphic flower or radially symmetrical flower. This is observed in the petunia, buttercup, and wild rose.
-Discrepancies in size or shape of the members of a whorl make the flower irregular such as in the canna and Asiatic dayflower.
-When a flower can be bisected by a single plane into two equal sections, it is a zygomorphic flower or bilaterally symmetrical flower. This is seen in the snapdragon, orchid, and sweet pea.
So, the correct answer is “Brassica”
Note: Trimerous symmetry is the characteristic in the monocotyledons such as maize, rice, coconut, etc. Pentamerous symmetry is the most common symmetry in the dicotyledons, however dimerous and tetramerous flowers are also seen in the dicotyledonous plants.
Complete answer:
Brassica has Actinomorphic flowers i.e it is a flower with radial symmetry. All the petals of brassica are similar in size and shape. The flower can be cut into two equal halves from any plane.
Additional information:

-A flower is supposed to be symmetrical when each whorl comprises an equal number of members or when the members of any one whorl are multiples of that before it.
-Thus, a symmetrical flower may have three sepals, three petals, three stamens, and three carpels, or the quantity of any of these members may be a multiple of three.
-A flower is dimerous in which the parts are in twos; trimerous, tetramerous, or pentamerous, in threes, fours, or fives, respectively.
-When the different parts of each whorl are identical, the flower is regular and is called as an actinomorphic flower or radially symmetrical flower. This is observed in the petunia, buttercup, and wild rose.
-Discrepancies in size or shape of the members of a whorl make the flower irregular such as in the canna and Asiatic dayflower.
-When a flower can be bisected by a single plane into two equal sections, it is a zygomorphic flower or bilaterally symmetrical flower. This is seen in the snapdragon, orchid, and sweet pea.
So, the correct answer is “Brassica”
Note: Trimerous symmetry is the characteristic in the monocotyledons such as maize, rice, coconut, etc. Pentamerous symmetry is the most common symmetry in the dicotyledons, however dimerous and tetramerous flowers are also seen in the dicotyledonous plants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




