
Question: Two types of $FXF$ angles are present in which of the following molecules $\left( {X = S,Xe,C} \right)$?
A: $S{F_4}$
B: $Xe{F_4}$
C: $S{F_6}$
D: $C{F_4}$
Answer
588k+ views
Hint: Bond angle is the angle that is formed between two bonds. For the formation of bond angle there must be at least three atoms and two bonds between these atoms. It is not necessary that bond angle is the same between all the bonds of a molecule.
Complete step by step answer:
In this question we have to attach a central atom with fluorine atoms so that the bond angle will be different for two pairs of bonds. To compare the bond angle there must be four bonds of the central atom with four fluorine atoms (as one bond angle is formed between two atoms).
$C{F_4}$ has tetrahedral geometry. In this structure all the bond angles are equal. This means $C{F_4}$ is not the answer as we have to find the molecule with two types of bond angles.
$S{F_6}$ has octahedral geometry. In this structure all the bond angles are equal. This means $S{F_6}$ is also not the answer as we have to find the molecule with two types of bond angles.
$Xe{F_4}$ also has tetrahedral geometry. In this structure all the bond angles are equal. This means $Xe{F_4}$ is also not the answer as we have to find the molecule with two types of bond angles.
$S{F_4}$ has seesaw geometry. In this structure all the bond angles are not equal. There are two bond angles in this geometry. This means $S{F_4}$ is the answer as we have to find the molecule with two types of bond angles.
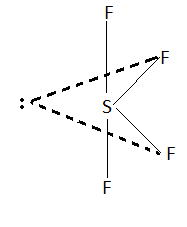
Structure of $S{F_4}$
So, the correct answer is option A .
Note:
Hybridization of $S{F_4}$ is $s{p^3}d$. Hybridization is intermixing of atomic orbitals of slightly different energies but of the same molecule to form new hybrid orbital whose energy will be same but different from parent orbitals.
Complete step by step answer:
In this question we have to attach a central atom with fluorine atoms so that the bond angle will be different for two pairs of bonds. To compare the bond angle there must be four bonds of the central atom with four fluorine atoms (as one bond angle is formed between two atoms).
$C{F_4}$ has tetrahedral geometry. In this structure all the bond angles are equal. This means $C{F_4}$ is not the answer as we have to find the molecule with two types of bond angles.
$S{F_6}$ has octahedral geometry. In this structure all the bond angles are equal. This means $S{F_6}$ is also not the answer as we have to find the molecule with two types of bond angles.
$Xe{F_4}$ also has tetrahedral geometry. In this structure all the bond angles are equal. This means $Xe{F_4}$ is also not the answer as we have to find the molecule with two types of bond angles.
$S{F_4}$ has seesaw geometry. In this structure all the bond angles are not equal. There are two bond angles in this geometry. This means $S{F_4}$ is the answer as we have to find the molecule with two types of bond angles.

Structure of $S{F_4}$
So, the correct answer is option A .
Note:
Hybridization of $S{F_4}$ is $s{p^3}d$. Hybridization is intermixing of atomic orbitals of slightly different energies but of the same molecule to form new hybrid orbital whose energy will be same but different from parent orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




