
Pyridine and pyrimidine are both aromatic compounds, how many electrons does each nitrogen atom contribute to the electron cloud of each compound?
Answer
545.1k+ views
Hint: When it comes to aromaticity, this question is asking us to consider which of the valence electrons of nitrogen count towards the Huckel's Rule. That is the \[4n+2\] rule. In order to solve this question, we will first start by drawing the structures of both the above compounds and later come to the conclusion from our observations. Nitrogen does not donate its lone pair for resonance.
Complete step-by-step answer:The structure of pyridine is as follows:
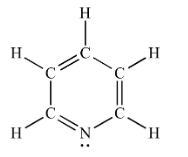
The structure of pyrimidine is as follows:
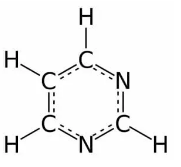
Within the $\pi$ electron cloud, the only ones delocalized are those in the ring, i.e., not in an orbital perpendicular to the $2 p {z}$ (vertical) orbitals of each carbon in the ring. Around each nitrogen, there are only three electron groups, indicating a trigonal planar geometry of electrons.
The XY-plane is perpendicular to the Z axis, since the \[s{{p}^{2}}\] orbitals are in the same plane as the rest of the molecule, and since the Z axis is vertical and the XY-plane is horizontal, the nitrogen \[s{{p}^{2}}\] orbital is perpendicular to each carbon's \[2pz\].
Therefore, the orbital $s p^{2}$ does not add its lone pair into the ring, and nitrogen only contributes electrons that are already bonded into the ring.
Therefore, each nitrogen contributes around three electrons of $\pi$ to the aromatic ring. If we assume that the carbon and nitrogen electronegativity are sufficiently similar, then there would be three electrons per nitrogen of $\pi$.
Note:Aromatic compounds are chemical compounds which consist of conjugated planar ring systems in place of individual alternating double and single bonds, accompanied by delocalized pi-electron clouds.
Complete step-by-step answer:The structure of pyridine is as follows:

The structure of pyrimidine is as follows:

Within the $\pi$ electron cloud, the only ones delocalized are those in the ring, i.e., not in an orbital perpendicular to the $2 p {z}$ (vertical) orbitals of each carbon in the ring. Around each nitrogen, there are only three electron groups, indicating a trigonal planar geometry of electrons.
The XY-plane is perpendicular to the Z axis, since the \[s{{p}^{2}}\] orbitals are in the same plane as the rest of the molecule, and since the Z axis is vertical and the XY-plane is horizontal, the nitrogen \[s{{p}^{2}}\] orbital is perpendicular to each carbon's \[2pz\].
Therefore, the orbital $s p^{2}$ does not add its lone pair into the ring, and nitrogen only contributes electrons that are already bonded into the ring.
Therefore, each nitrogen contributes around three electrons of $\pi$ to the aromatic ring. If we assume that the carbon and nitrogen electronegativity are sufficiently similar, then there would be three electrons per nitrogen of $\pi$.
Note:Aromatic compounds are chemical compounds which consist of conjugated planar ring systems in place of individual alternating double and single bonds, accompanied by delocalized pi-electron clouds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




