
How to put chain numbers?
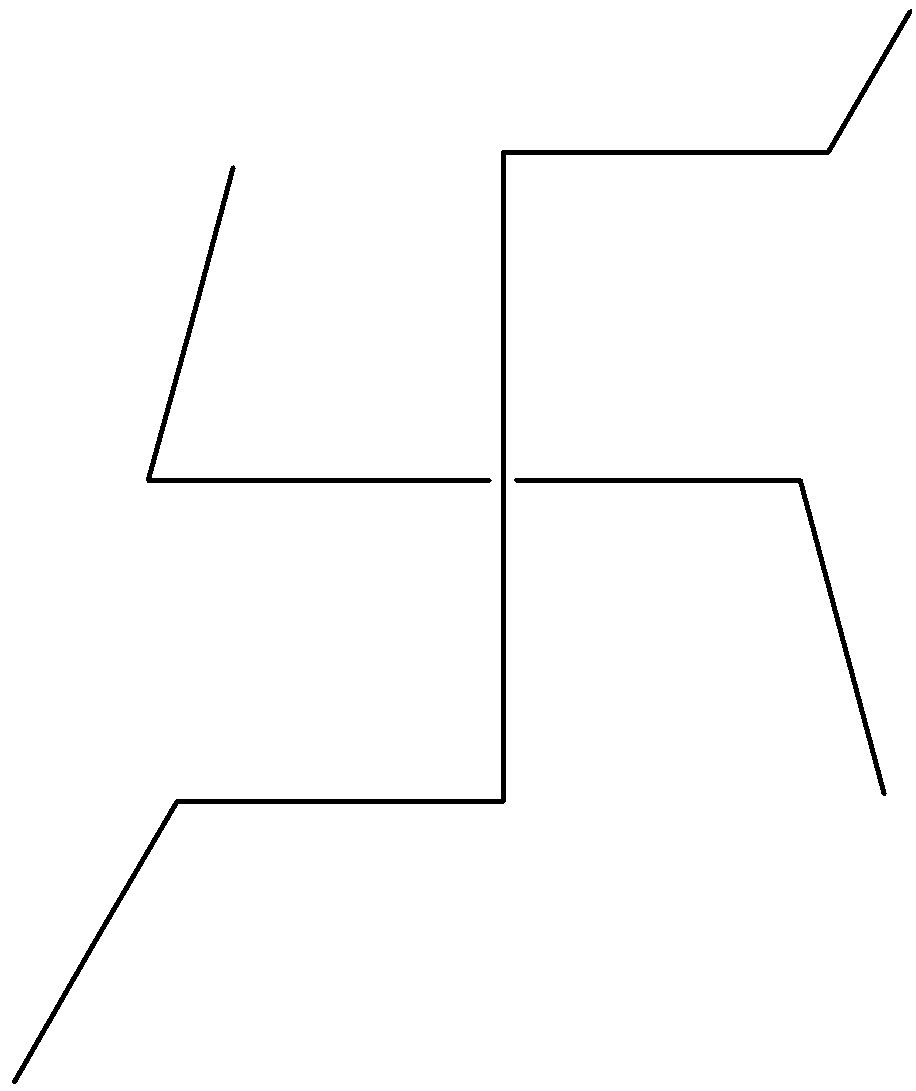
$4,4$- Diethyl-heptane

Answer
560.7k+ views
Hint: We should always follow the nomenclature while writing IUPAC names of hydrocarbons. Nomenclature is the set of rules or systems of naming organic compounds. For naming organic compounds there are some sets of rules by which we can name the above compound properly as $4,4$- Diethyl-heptane.
Complete step by step answer:
Here we will discuss the rules of nomenclature of saturated hydrocarbons. Using the set of rules we will also come to know how we can put chain numbers in $4,4$- Diethyl-heptane. So, now we will study the rule used to put chain numbers.
The rule used to put a chain number on saturated hydrocarbon is called the longest chain rule. It is suggested that while naming a saturated hydrocarbon we should select the longest continuous chain of carbon atoms in the molecule. The selected chain is also called the principal chain or parent chain.
Now let us consider the structure of $4,4$- Ethyl-heptane as we need to put a chain number on it. There are several possibilities to put a chain number on the given $4,4$- Diethyl-heptane.
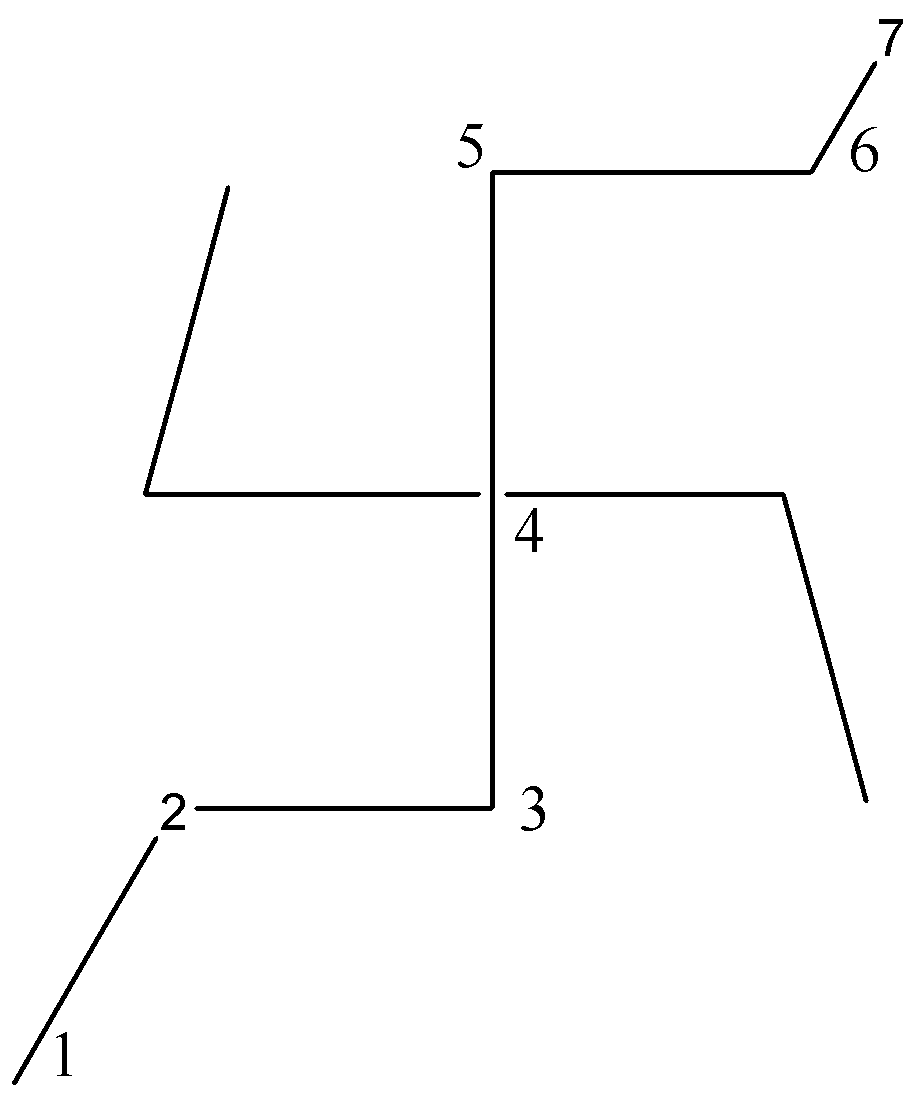
The first possibility is shown below where the number of carbon atoms in a chain is $7$.
Another possibility of putting chain number on the given saturated hydrocarbon is
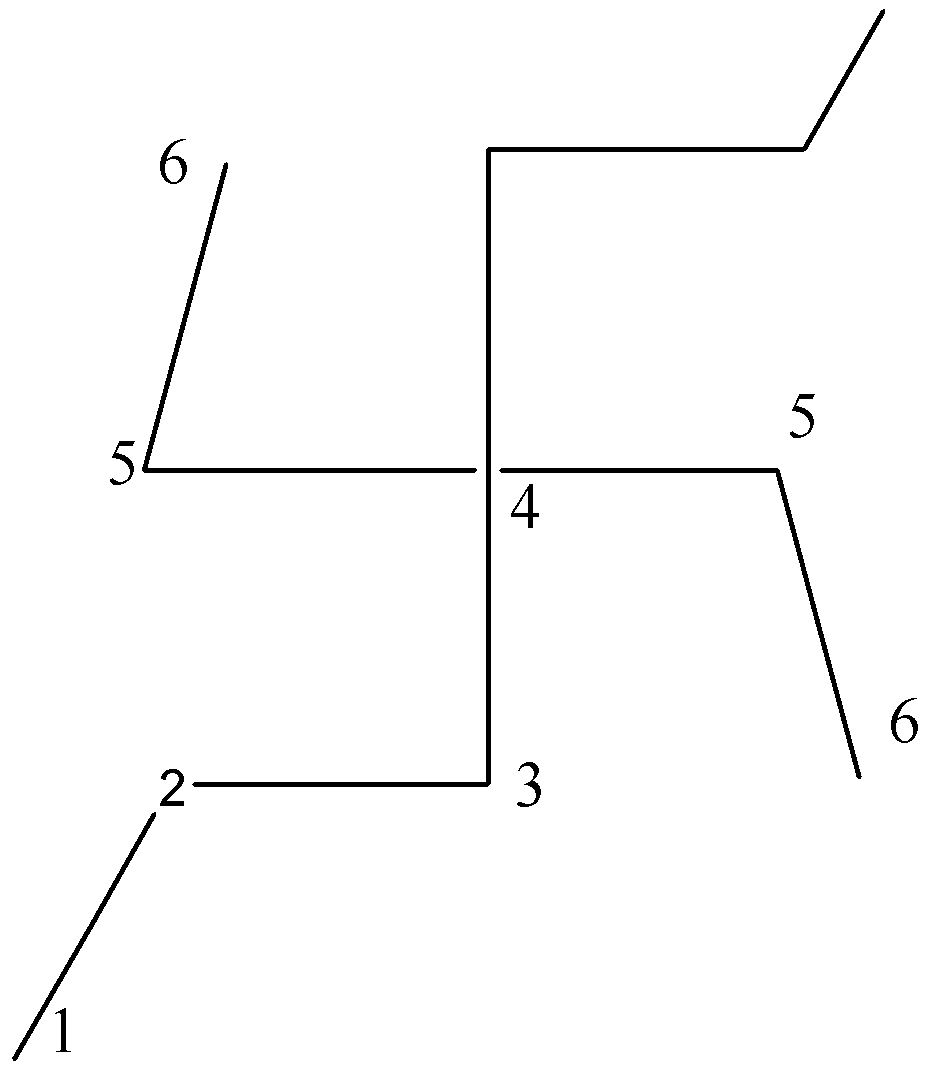
We can observe that there are $6$ carbon atoms in the considered parent chain.
Another possibility of putting a chain number on the given saturated hydrocarbon is
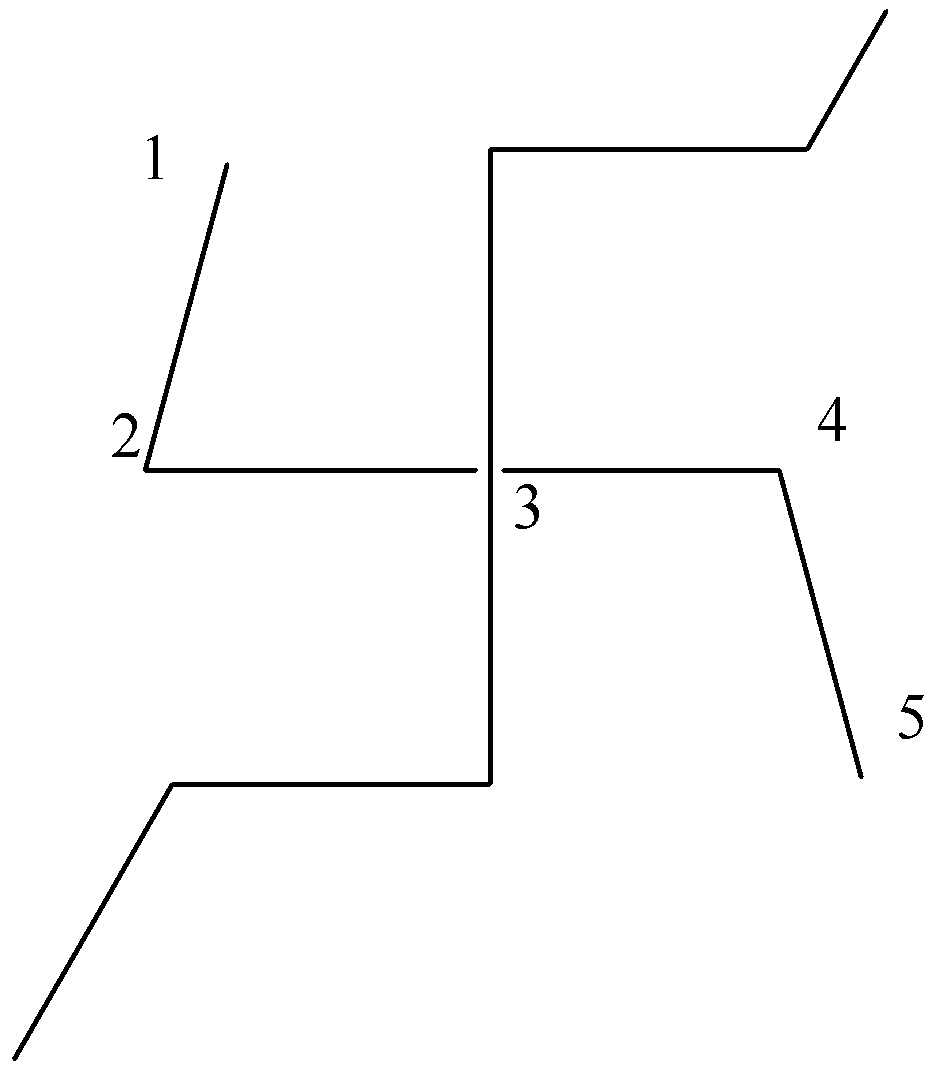
In this compound, we can observe that considered parent chains have $5$ a carbon atom.
Conclusion: Out of the three possibilities we will consider the first possibility in which the parent chain is the longest having $7$ carbon atoms.
Note: It should be noted that the carbon atoms which are not included in this chain are considered as substituents.
If two chains of equal lengths are possible, then one with more number side chains will be considered as the parent chain.
Complete step by step answer:
Here we will discuss the rules of nomenclature of saturated hydrocarbons. Using the set of rules we will also come to know how we can put chain numbers in $4,4$- Diethyl-heptane. So, now we will study the rule used to put chain numbers.
The rule used to put a chain number on saturated hydrocarbon is called the longest chain rule. It is suggested that while naming a saturated hydrocarbon we should select the longest continuous chain of carbon atoms in the molecule. The selected chain is also called the principal chain or parent chain.
Now let us consider the structure of $4,4$- Ethyl-heptane as we need to put a chain number on it. There are several possibilities to put a chain number on the given $4,4$- Diethyl-heptane.
The first possibility is shown below where the number of carbon atoms in a chain is $7$.

Another possibility of putting chain number on the given saturated hydrocarbon is

We can observe that there are $6$ carbon atoms in the considered parent chain.
Another possibility of putting a chain number on the given saturated hydrocarbon is

In this compound, we can observe that considered parent chains have $5$ a carbon atom.
Conclusion: Out of the three possibilities we will consider the first possibility in which the parent chain is the longest having $7$ carbon atoms.
Note: It should be noted that the carbon atoms which are not included in this chain are considered as substituents.
If two chains of equal lengths are possible, then one with more number side chains will be considered as the parent chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




