
Propyl amine and aniline can be distinguished by azo dye test.
A. True
B. False
Answer
576.9k+ views
Hint: We should know what azo dye test and for what it is used. The azo dye test is used to distinguish amines. The amines are distinguished based on the formation of azo compounds. The azo compounds show colours.
Complete Answer :
The functional group amine is represented as:
$ - {\text{N}}{{\text{H}}_{\text{2}}}$. The ${\text{R}} - {\text{N}}{{\text{H}}_2}$is known as an amine. Here, R can be alkyl then the amine is known as aliphatic amine and if the R is aryl group then the amine is known as an aromatic amine.
On the basis of attachments amine are of three types:
Primary amine having $ - {\text{N}}{{\text{H}}_2}$ group.
Secondary amine having $ - {\text{NH}}\, - $ group.
Tertiary amine having $ - \mathop {\text{N}}\limits_| - $ group.
The primary, secondary and tertiary amine have different chemical and physical properties and hence different reactivates.
So, different tests are used to differentiate the primary, secondary and tertiary amines.
The azo dye test is used to distinguish aromatic and aliphatic amines.
In this test, amines are reacted with nitrous acid, so a diazonium salt forms. The ${{\text{N}}_{\text{2}}}$ of diazonium salt of aromatic amine act as an electrophile so, another aromatic amine attacks on this electrophile and ${{\text{N}}_{\text{2}}}$get bridged between two aromatic amines. The azo compounds show colour hence used as a dye. Thus the test is known as azo dye test.So, we will find the aromatic amine.
The structure of the given amines are as follows:
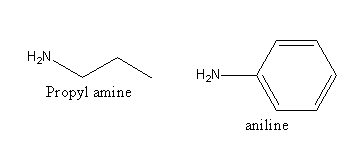
Propyl amine is aliphatic and aniline is an aromatic amine. So, aniline will form the azo compound and propylamine do not, so both can be distinguished by the azo dye test. In the diazo test, aniline is reacted with nitrous acid then beta-naphthol.
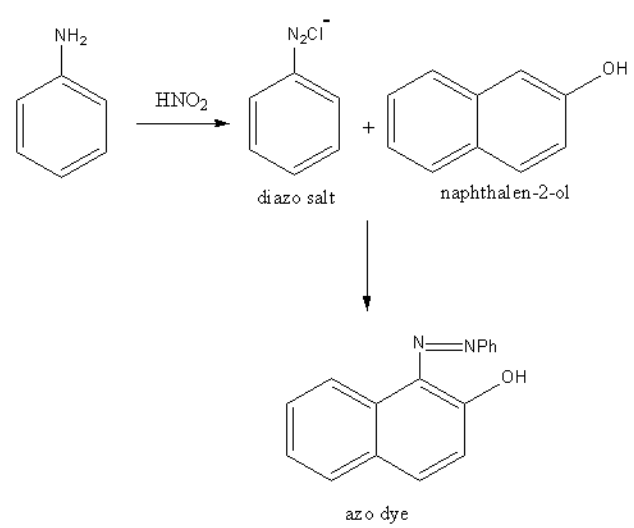
The formed azo dye has an orange colour.
So, it is true that propyl amine and aniline can be distinguished by the azo dye test.
Therefore, option (A) true, is correct.
Note: The test used to differentiate amine are the carbylamine test, nitrous acid test, solubility, and litmus test, Hinsberg, and azo dye test. Litmus and solubility tests are used to determine the presence of an amine. Carbylamine, nitrous acid and Hinsberg test are used to differentiate primary, secondary and tertiary amines. An azo dye test is used to differentiate aliphatic and aromatic amines.
Complete Answer :
The functional group amine is represented as:
$ - {\text{N}}{{\text{H}}_{\text{2}}}$. The ${\text{R}} - {\text{N}}{{\text{H}}_2}$is known as an amine. Here, R can be alkyl then the amine is known as aliphatic amine and if the R is aryl group then the amine is known as an aromatic amine.
On the basis of attachments amine are of three types:
Primary amine having $ - {\text{N}}{{\text{H}}_2}$ group.
Secondary amine having $ - {\text{NH}}\, - $ group.
Tertiary amine having $ - \mathop {\text{N}}\limits_| - $ group.
The primary, secondary and tertiary amine have different chemical and physical properties and hence different reactivates.
So, different tests are used to differentiate the primary, secondary and tertiary amines.
The azo dye test is used to distinguish aromatic and aliphatic amines.
In this test, amines are reacted with nitrous acid, so a diazonium salt forms. The ${{\text{N}}_{\text{2}}}$ of diazonium salt of aromatic amine act as an electrophile so, another aromatic amine attacks on this electrophile and ${{\text{N}}_{\text{2}}}$get bridged between two aromatic amines. The azo compounds show colour hence used as a dye. Thus the test is known as azo dye test.So, we will find the aromatic amine.
The structure of the given amines are as follows:

Propyl amine is aliphatic and aniline is an aromatic amine. So, aniline will form the azo compound and propylamine do not, so both can be distinguished by the azo dye test. In the diazo test, aniline is reacted with nitrous acid then beta-naphthol.

The formed azo dye has an orange colour.
So, it is true that propyl amine and aniline can be distinguished by the azo dye test.
Therefore, option (A) true, is correct.
Note: The test used to differentiate amine are the carbylamine test, nitrous acid test, solubility, and litmus test, Hinsberg, and azo dye test. Litmus and solubility tests are used to determine the presence of an amine. Carbylamine, nitrous acid and Hinsberg test are used to differentiate primary, secondary and tertiary amines. An azo dye test is used to differentiate aliphatic and aromatic amines.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

What is myopia and hypermetropia How are they corrected class 12 physics CBSE




