
How many products will be formed in the following reaction?
$C{{H}_{3}}-Cl\ +\ C{{H}_{3}}-C{{H}_{2}}-Cl\xrightarrow[dry\ ether]{Na}$
A. $1$
B. $2$
C. $3$
D. $4$
Answer
516.6k+ views
Hint: Free radical: When homolytic cleavage of a covalent bond takes place, then the electrons are distributed among atoms in such a manner that after cleavage, each atom has an unshared electron which is termed as free radical. Generally, it shows properties similar to the properties of cations.
Complete answer:
Wurtz reaction: It is a type of organic reaction in which sodium metal in presence of dry ether reacts with two moles of alkyl halides, to yield higher alkanes along with sodium halide compound. The Wurtz reaction follows a free radical mechanism i.e., homolytic cleavage of carbon halide bonds takes place during the reaction.
For the given reaction, there are two different alkyl halide compounds taken. So, there are three cases in which the reaction may proceed. The cases are as follows:
Case-1: Methyl chloride reacts with itself to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:

Step-2: Formation of ethane:
$CH_{3}^{\bullet }\,\,+\,\,CH_{3}^{\bullet }\to C{{H}_{3}}-C{{H}_{3}}$
Case-2: Ethyl chloride reacts with itself to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:
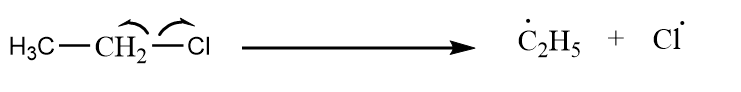
Step-2: Formation of butane:
${{C}_{2}}H_{5}^{\bullet }\,\,+\,\,{{C}_{2}}H_{5}^{\bullet }\to {{C}_{2}}{{H}_{5}}-{{C}_{2}}{{H}_{5}}$
Case-3: Ethyl chloride reacts with methyl chloride to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:
Cleavage of carbon chlorine bond in ethyl chloride: -
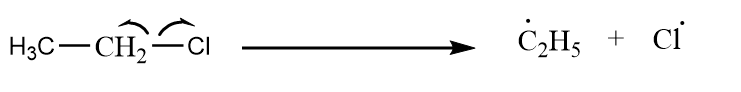
Cleavage of carbon chlorine bond in methyl chloride: -

Step-2: Formation of propane:
${{C}_{2}}H_{5}^{\bullet }\,\,+\,\,CH_{3}^{\bullet }\to {{C}_{2}}{{H}_{5}}-C{{H}_{3}}$
Hence, for the given reaction number of products formed $=3$.
Thus, option (C) is the correct answer.
Note:
It is important to note that Wurtz reaction is one of the important reactions in organic chemistry to yield higher alkanes. Apart from sodium metal, metals like silver, zinc and iron can also be used in these reactions. But if the tertiary alkyl halide is taken as the reactant, then the Wurtz reaction does not take place due to steric hindrance.
Complete answer:
Wurtz reaction: It is a type of organic reaction in which sodium metal in presence of dry ether reacts with two moles of alkyl halides, to yield higher alkanes along with sodium halide compound. The Wurtz reaction follows a free radical mechanism i.e., homolytic cleavage of carbon halide bonds takes place during the reaction.
For the given reaction, there are two different alkyl halide compounds taken. So, there are three cases in which the reaction may proceed. The cases are as follows:
Case-1: Methyl chloride reacts with itself to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:

Step-2: Formation of ethane:
$CH_{3}^{\bullet }\,\,+\,\,CH_{3}^{\bullet }\to C{{H}_{3}}-C{{H}_{3}}$
Case-2: Ethyl chloride reacts with itself to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:

Step-2: Formation of butane:
${{C}_{2}}H_{5}^{\bullet }\,\,+\,\,{{C}_{2}}H_{5}^{\bullet }\to {{C}_{2}}{{H}_{5}}-{{C}_{2}}{{H}_{5}}$
Case-3: Ethyl chloride reacts with methyl chloride to give product via Wurtz reaction.
Step-1: Homolytic cleavage of alkyl halide:
Cleavage of carbon chlorine bond in ethyl chloride: -

Cleavage of carbon chlorine bond in methyl chloride: -

Step-2: Formation of propane:
${{C}_{2}}H_{5}^{\bullet }\,\,+\,\,CH_{3}^{\bullet }\to {{C}_{2}}{{H}_{5}}-C{{H}_{3}}$
Hence, for the given reaction number of products formed $=3$.
Thus, option (C) is the correct answer.
Note:
It is important to note that Wurtz reaction is one of the important reactions in organic chemistry to yield higher alkanes. Apart from sodium metal, metals like silver, zinc and iron can also be used in these reactions. But if the tertiary alkyl halide is taken as the reactant, then the Wurtz reaction does not take place due to steric hindrance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




