
Products forms by following reactions are
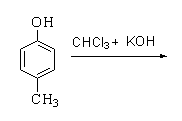
A.
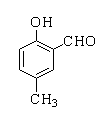
B.
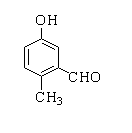
C.
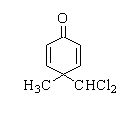
D.
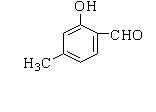





Answer
578.4k+ views
Hint:The given reaction is a type of substitution reaction. This reaction is known as the Reimer Tiemann’s reaction named after the chemist Carl Reimer and Ferdinand Tiemann. In this reaction, the ortho-formylation of phenol takes place.
Complete step by step answer:
In Reimer Tiemann reaction, when a phenol $({C_6}{H_5}OH)$ is reacted with chloroform$(CHC{l_3})$ in the availability of a base sodium hydroxide (NaOH), an aldehydic group (-CHO) gets attached to the ortho position (o-) of the benzene ring. The resulting compound formed is o-hydroxybenzaldehyde.
The general Reimer Tiemann reaction is shown below.
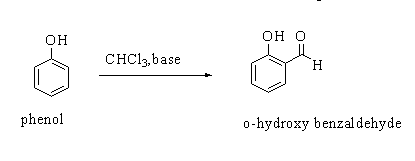
The compound o-hydroxybenzaldehyde is commonly known as salicylaldehyde.
The given reaction is shown below.
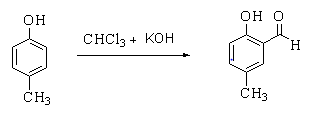
In this reaction, p-cresol is treated with chloroform in the presence of a base to form a 2-hydroxy-5-methylbenzaldehyde.
In this reaction, the first deprotonation of chloroform takes place with the help of base forming a chloroform carbanion. The chloroform carbanion easily experiences alpha elimination forming a dichlorocarbene. The base also deprotonates the phenol forming a negatively charged phenoxide. The negative ion is delocalized by the benzene ring making it more nucleophilic. The nucleophile then attacks the dichlorocarbene to form an intermediate dichloromethyl substituted phenol. The intermediate finally undergoes basic hydrolysis to form 2-hydroxy-5-methylbenzaldehyde.
Therefore, the correct option is A.
Note:
When phenol is converted to ortho-hydroxybenzaldehyde with the help of chloroform, base, and acid workup. It should be highly considered that the carbene group is highly electron-deficient because of the two chlorine groups has the electron-withdrawing nature, therefore gets attracted towards the phenoxide ion rich with electrons to favor the ortho-formylation.
Complete step by step answer:
In Reimer Tiemann reaction, when a phenol $({C_6}{H_5}OH)$ is reacted with chloroform$(CHC{l_3})$ in the availability of a base sodium hydroxide (NaOH), an aldehydic group (-CHO) gets attached to the ortho position (o-) of the benzene ring. The resulting compound formed is o-hydroxybenzaldehyde.
The general Reimer Tiemann reaction is shown below.

The compound o-hydroxybenzaldehyde is commonly known as salicylaldehyde.
The given reaction is shown below.

In this reaction, p-cresol is treated with chloroform in the presence of a base to form a 2-hydroxy-5-methylbenzaldehyde.
In this reaction, the first deprotonation of chloroform takes place with the help of base forming a chloroform carbanion. The chloroform carbanion easily experiences alpha elimination forming a dichlorocarbene. The base also deprotonates the phenol forming a negatively charged phenoxide. The negative ion is delocalized by the benzene ring making it more nucleophilic. The nucleophile then attacks the dichlorocarbene to form an intermediate dichloromethyl substituted phenol. The intermediate finally undergoes basic hydrolysis to form 2-hydroxy-5-methylbenzaldehyde.
Therefore, the correct option is A.
Note:
When phenol is converted to ortho-hydroxybenzaldehyde with the help of chloroform, base, and acid workup. It should be highly considered that the carbene group is highly electron-deficient because of the two chlorine groups has the electron-withdrawing nature, therefore gets attracted towards the phenoxide ion rich with electrons to favor the ortho-formylation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




