
How many products are obtained in the given reaction.
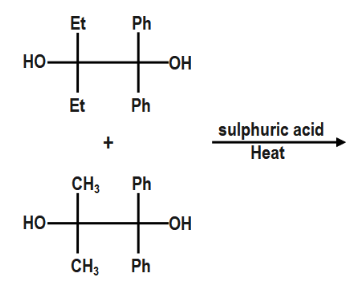
(A) $ 1 $
(B) $ 2 $
(C) $ 3 $
(D) $ 4 $

Answer
529.2k+ views
Hint :In the given reaction if we look at the reactants, we see that they are deol's that is two alcohol groups attached with each compound. The reagent is sulfuric acid. Now, if you are able to recall that when diol reacts with sulfuric acid, then this reaction has a famous name that is pinacol-pinacolone reaction. So, let us see this reaction and find out the products of the given reaction.
Complete Step By Step Answer:
Let us firstly understand what do we mean by the term pinacol and pinacolone.
A pinacol is a compound in which two hydroxyl groups are present and each hydroxyl group is attached to a vicinal carbon atom. It is a white solid organic compound.
A pinacolone is basically a ketone. It spears as a colorless liquid and has a camphor like smell.
In this reaction, each pinacol will react with sulfuric acid and will yield a pinacolone. So, at the end there will be two pinacolones as the product of the reaction.
Let us see the first compound. Now, if we look at the mechanism of the reaction.
Since, we can see that the reaction is occurring in an acidic medium, the acid will protonate the hydroxyl group of the pinacol. The reaction is as follows.
After this protonation, water molecules are lost and a carbocation is generated. Now if we look at the carbocation above, we will find that at this position carbocation will be less stable. If the carbocation would have been generated in the carbon to which $ OH $ is attached, then the resonance of electrons of Oxygen from $ OH $ group would stabilize the carbocation more.
So, there will be an ethyl shift. Ethyl group will shift to the adjacent carbon and carbocation will be generated in carbon attached with $ -OH.~ $ The reaction is as follows. The carbocation thus formed is more stable than that formed.
The oxygen atom of $ OH $ bonded to carbon atoms now gets deprotonated and thus as a result a ketone (pinacolone) is formed. The reaction is as follows.
So, the above pinacolone is the product of the reaction of sulfuric acid with the first pinacol given in the question. Another pinacol will react in the similar manner through the same mechanism to give another pinacolone. The only difference is that there is a methyl group attached, and so the methyl shift will occur. The final equation of reaction of acid with another pinacol given in the question is;
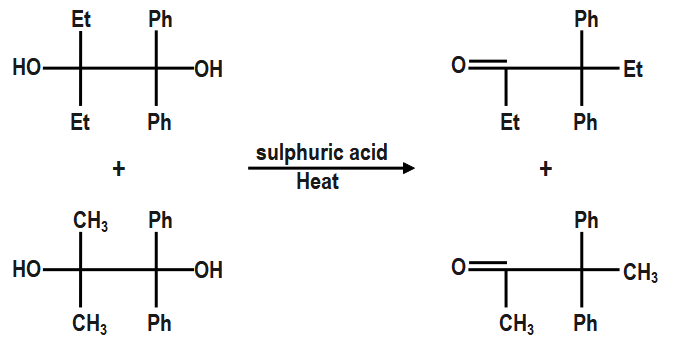
Therefore, the final two pinacolones formed are as follows.
Hence, the correct option is Option B.
Note :
In the above reaction, the formation of carbocation is the rate determining step. A carbocation is an intermediate in this reaction and we know that in any reaction mechanism if the intermediate is stable then the products themselves are stable enough. This is the only reason why the ethyl shift in the first pinacol and methyl shift in the second pinacol will occur, so that carbocation gets more and more stable.
Complete Step By Step Answer:
Let us firstly understand what do we mean by the term pinacol and pinacolone.
A pinacol is a compound in which two hydroxyl groups are present and each hydroxyl group is attached to a vicinal carbon atom. It is a white solid organic compound.
A pinacolone is basically a ketone. It spears as a colorless liquid and has a camphor like smell.
In this reaction, each pinacol will react with sulfuric acid and will yield a pinacolone. So, at the end there will be two pinacolones as the product of the reaction.
Let us see the first compound. Now, if we look at the mechanism of the reaction.
Since, we can see that the reaction is occurring in an acidic medium, the acid will protonate the hydroxyl group of the pinacol. The reaction is as follows.
After this protonation, water molecules are lost and a carbocation is generated. Now if we look at the carbocation above, we will find that at this position carbocation will be less stable. If the carbocation would have been generated in the carbon to which $ OH $ is attached, then the resonance of electrons of Oxygen from $ OH $ group would stabilize the carbocation more.
So, there will be an ethyl shift. Ethyl group will shift to the adjacent carbon and carbocation will be generated in carbon attached with $ -OH.~ $ The reaction is as follows. The carbocation thus formed is more stable than that formed.
The oxygen atom of $ OH $ bonded to carbon atoms now gets deprotonated and thus as a result a ketone (pinacolone) is formed. The reaction is as follows.
So, the above pinacolone is the product of the reaction of sulfuric acid with the first pinacol given in the question. Another pinacol will react in the similar manner through the same mechanism to give another pinacolone. The only difference is that there is a methyl group attached, and so the methyl shift will occur. The final equation of reaction of acid with another pinacol given in the question is;

Therefore, the final two pinacolones formed are as follows.
Hence, the correct option is Option B.
Note :
In the above reaction, the formation of carbocation is the rate determining step. A carbocation is an intermediate in this reaction and we know that in any reaction mechanism if the intermediate is stable then the products themselves are stable enough. This is the only reason why the ethyl shift in the first pinacol and methyl shift in the second pinacol will occur, so that carbocation gets more and more stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




