
Product A in the below reaction is:
${C_6}{H_5}N{H_2}\,\, + \,\,CHC{l_3}\,\, + \,KOH\, \to \,A\, + \,KCl\, + {H_2}O$
A.

B.
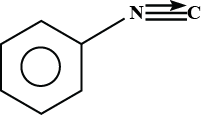
C.
D.




Answer
569.4k+ views
Hint:
In this reaction. We have to identify the product A .In the reaction Aniline, chloroform and alcoholic potassium hydroxide on heating gives a foul smelling compound .
Complete step by step answer:
The given reaction is an example of isocyanide test , also known as Carbylamine reaction . When aliphatic or aromatic ${1^ \circ }$ amines reacts with chloroform in the presence of strong alkali then a foul smelling compound isocyanide is formed . The reaction which takes place is :
${C_6}{H_5}N{H_2}\,\, + \,\,CHC{l_3}\,\, + \,3KOH\, \to \,{C_6}\,{H_5}NC + \,3KCl\, + 3{H_2}O$
In this reaction phenyl isocyanide is formed which gives offensive smell .
Isocyanide test is used to distinguish primary amines from secondary and tertiary amines , as secondary and tertiary amines do not form foul smelling compounds when they react with chloroform and alcoholic potassium hydroxide .
The mechanism which takes place in the above reaction is described below .
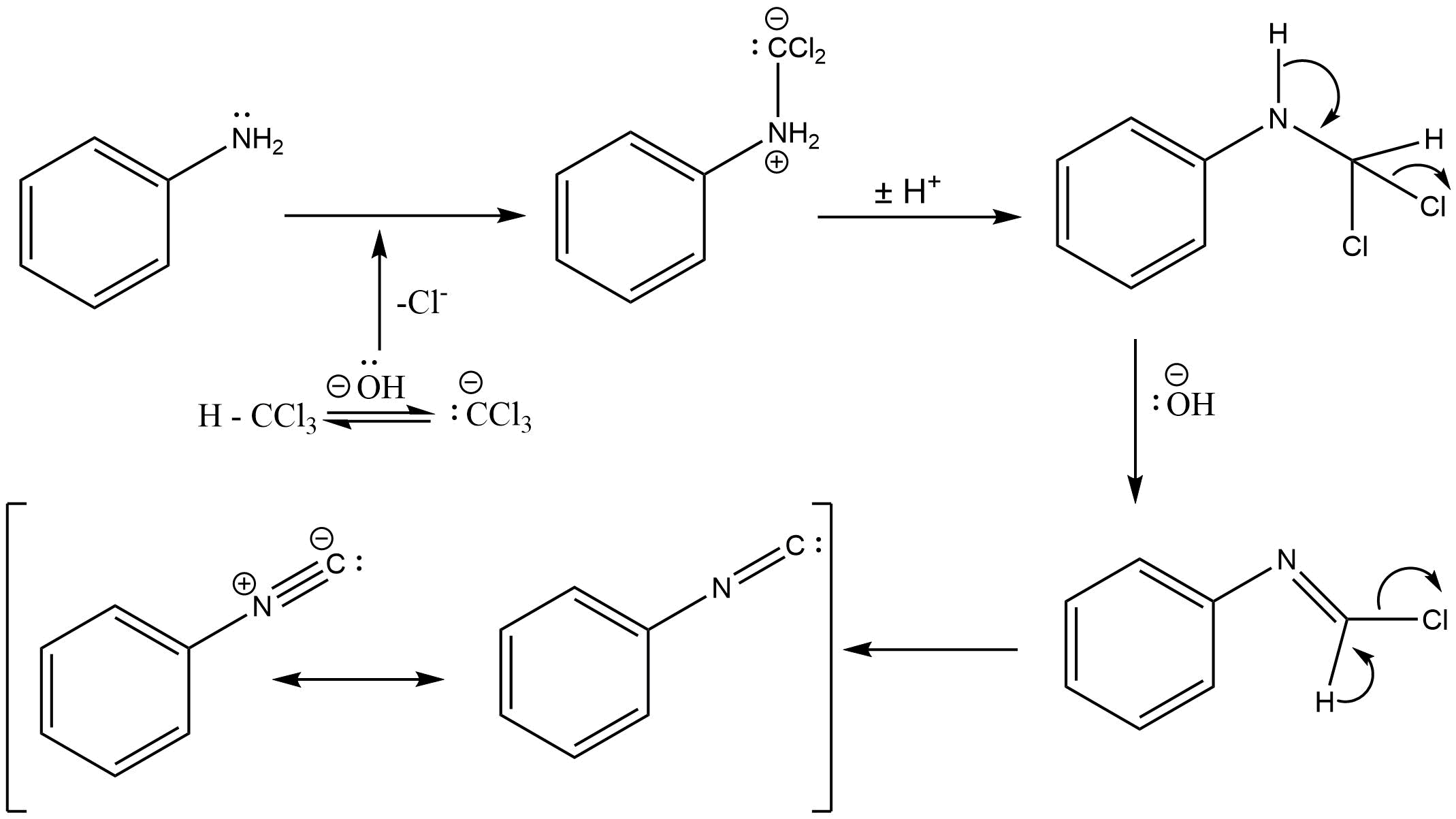
As you can see in the given figure , first aniline reacts with dichlorocarbene ( which is formed from dehydrohalogenation of chloroform ) , then the intermediate which is formed is very reactive . The attack of electrophilic dichlorocarbene takes place on the nucleophilic centre of the primary amine , finally hydrochloric acid is eliminated from the compound and isonitrile is formed .
Hence , the product formed is phenyl isocyanide .
So , option B is correct .
Note:We can use the carbylamine reaction to synthesize isocyanides using primary amines and reacting them with chloroform and a base . We can also use this test to check for the presence of a primary amine in a given substrate .
In this reaction. We have to identify the product A .In the reaction Aniline, chloroform and alcoholic potassium hydroxide on heating gives a foul smelling compound .
Complete step by step answer:
The given reaction is an example of isocyanide test , also known as Carbylamine reaction . When aliphatic or aromatic ${1^ \circ }$ amines reacts with chloroform in the presence of strong alkali then a foul smelling compound isocyanide is formed . The reaction which takes place is :
${C_6}{H_5}N{H_2}\,\, + \,\,CHC{l_3}\,\, + \,3KOH\, \to \,{C_6}\,{H_5}NC + \,3KCl\, + 3{H_2}O$
In this reaction phenyl isocyanide is formed which gives offensive smell .
Isocyanide test is used to distinguish primary amines from secondary and tertiary amines , as secondary and tertiary amines do not form foul smelling compounds when they react with chloroform and alcoholic potassium hydroxide .
The mechanism which takes place in the above reaction is described below .

As you can see in the given figure , first aniline reacts with dichlorocarbene ( which is formed from dehydrohalogenation of chloroform ) , then the intermediate which is formed is very reactive . The attack of electrophilic dichlorocarbene takes place on the nucleophilic centre of the primary amine , finally hydrochloric acid is eliminated from the compound and isonitrile is formed .
Hence , the product formed is phenyl isocyanide .
So , option B is correct .
Note:We can use the carbylamine reaction to synthesize isocyanides using primary amines and reacting them with chloroform and a base . We can also use this test to check for the presence of a primary amine in a given substrate .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




