
Product of the reaction is:
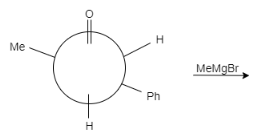
A.
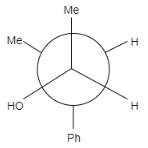
B.
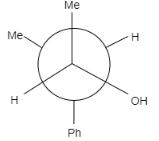
C. Both (A) and (B)
D. None of these



Answer
555.6k+ views
Hint: Grignard reagent is defined as a strong nucleophile that has a chemical formula \[RMgX\]. Here, $R$ is the alkyl group and $X$ is any halogen. Grignard reagent can behave as a carbonyl compound with electrophiles. It is used to form carbon- carbon bonds somewhat similar to organolithium.
Complete step-by-step answer:
Grignard reagent is prepared when magnesium is reacted with alkyl halides or aryl halides and form Grignard reagent. We can make these reagents with alkyl chlorides and alkyl bromides but not with alkyl fluorides. The solvent used for the synthesis of Grignard reagent is diethyl ether. The magnesium halide part of the reagent acts as a carbanion whereas the alkyl group acts as a cation.
Let us discuss the Grignard reaction mechanism-
The Grignard reagent which is nucleophilic attacks the electrophilic carbon of the carbonyl group. The Grignard reagent acts as a base therefore it attacks on the proton of the carbonyl compound. After that, the action of the Grignard reagent attacks on the carbon of the carbonyl group whereas the anion attacks on the oxygen atom.
In this question, the carbonyl compound is planar and shows $s{{p}^{2}}$ geometry. When Grignard reagents $M{{e}^{\delta +}}M{{g}^{-}}Br$ attacks on the given staggered compound, the attack can take place from any side. The probability of the attack of a nucleophile is equal for both the compounds.
Let us see the reaction of both the compounds-
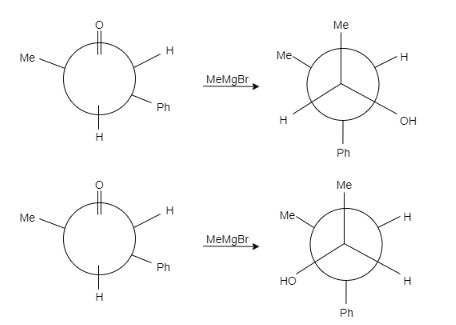
Therefore, formation of both the products is possible.
Hence the correct answer is C.
Note: It is to note that the major product formed is (b) because the hydroxyl group is present between the hydrogen group and phenyl group that means it is sterically less hindered whereas in option (a) the hydroxyl group is present in between the methyl and phenyl group, that means it will show more steric hindrance than (a) and minor product is formed.
Complete step-by-step answer:
Grignard reagent is prepared when magnesium is reacted with alkyl halides or aryl halides and form Grignard reagent. We can make these reagents with alkyl chlorides and alkyl bromides but not with alkyl fluorides. The solvent used for the synthesis of Grignard reagent is diethyl ether. The magnesium halide part of the reagent acts as a carbanion whereas the alkyl group acts as a cation.
Let us discuss the Grignard reaction mechanism-
The Grignard reagent which is nucleophilic attacks the electrophilic carbon of the carbonyl group. The Grignard reagent acts as a base therefore it attacks on the proton of the carbonyl compound. After that, the action of the Grignard reagent attacks on the carbon of the carbonyl group whereas the anion attacks on the oxygen atom.
In this question, the carbonyl compound is planar and shows $s{{p}^{2}}$ geometry. When Grignard reagents $M{{e}^{\delta +}}M{{g}^{-}}Br$ attacks on the given staggered compound, the attack can take place from any side. The probability of the attack of a nucleophile is equal for both the compounds.
Let us see the reaction of both the compounds-

Therefore, formation of both the products is possible.
Hence the correct answer is C.
Note: It is to note that the major product formed is (b) because the hydroxyl group is present between the hydrogen group and phenyl group that means it is sterically less hindered whereas in option (a) the hydroxyl group is present in between the methyl and phenyl group, that means it will show more steric hindrance than (a) and minor product is formed.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




