
What will be product in given reaction
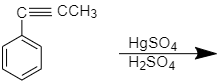
A.
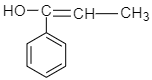
B.
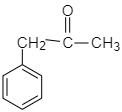
C.

D.
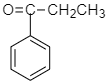
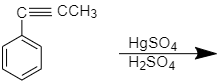




Answer
510.6k+ views
Hint: Aldehydes and ketones can be prepared by the hydration of alkynes in the presence of mercuric salts as catalyst, Hydration of alkynes other than acetylene gives ketone and also Formaldehyde cannot be prepared by the hydration of alkynes method.
Aliphatic aldehydes and ketones are the functional isomers of each other because they have the same molecular formula but different functional groups.
Complete answer:
The addition of water to alkynes forms unstable enol (intermediate in organic chemistry that is represented as an alkene with a hydroxyl group attached to one end of alkene double bond) which rearranges to form a more stable keto tautomer. The mechanism of the reaction is as follows,
At the first step, the $ \pi $ electron of the triple bond behaves as a Lewis base and attacks the proton, protonating the carbon with hydrogen substituents, and then there is an attack of a nucleophilic water molecule on electrophilic carbocation, this creates an Oxonium ion.
Then the base is deprotonated giving rise to alcohol called enol then occurs tautomerism i.e. concurrent proton and double bond shift, which goes from enol form to keto isomer form.
According to the question prop-1-enyl benzene in the presence of $ HgS{O_4} $ forms 1-phenylprop-1-en-1-ol which on further tautomerization forms propiophenone.
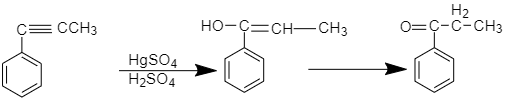
Therefore the correct answer is option C.
Note:
Hydroboration-oxidation method is complementary to the direct $ H{g^{2 + }} $ catalysed hydration reaction of terminal alkynes because different products are obtained.
Hydration of terminal alkyne with $ H{g^{2 + }} $ salt and water gives methyl ketone whereas hydroboration-oxidation of the same terminal alkyne gives aldehyde.
Aliphatic aldehydes and ketones are the functional isomers of each other because they have the same molecular formula but different functional groups.
Complete answer:
The addition of water to alkynes forms unstable enol (intermediate in organic chemistry that is represented as an alkene with a hydroxyl group attached to one end of alkene double bond) which rearranges to form a more stable keto tautomer. The mechanism of the reaction is as follows,
At the first step, the $ \pi $ electron of the triple bond behaves as a Lewis base and attacks the proton, protonating the carbon with hydrogen substituents, and then there is an attack of a nucleophilic water molecule on electrophilic carbocation, this creates an Oxonium ion.
Then the base is deprotonated giving rise to alcohol called enol then occurs tautomerism i.e. concurrent proton and double bond shift, which goes from enol form to keto isomer form.
According to the question prop-1-enyl benzene in the presence of $ HgS{O_4} $ forms 1-phenylprop-1-en-1-ol which on further tautomerization forms propiophenone.

Therefore the correct answer is option C.
Note:
Hydroboration-oxidation method is complementary to the direct $ H{g^{2 + }} $ catalysed hydration reaction of terminal alkynes because different products are obtained.
Hydration of terminal alkyne with $ H{g^{2 + }} $ salt and water gives methyl ketone whereas hydroboration-oxidation of the same terminal alkyne gives aldehyde.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




