
What is the product B in the following reaction:
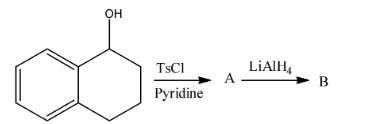
A.
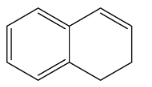
B.
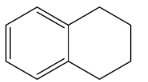
C.
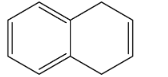
D.
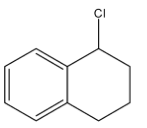





Answer
538.5k+ views
Hint: TsCl is called p-toluenesulfonyl chloride. TSCl reacts with primary or secondary alcohols and forms tosylate. These tosylates are going to act as good leaving groups in the presence of strong reducing agents.
Complete step by step solution:
- In the question it is given to find the product B in the given chemical reaction.
- The given chemical reaction contains two steps.
Step-1:
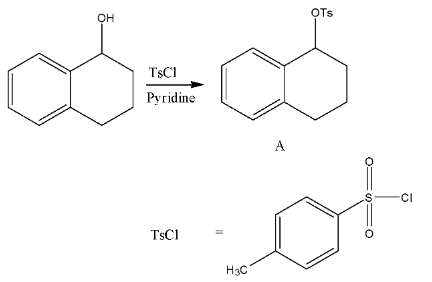
- In the first step TsCl = p-toluenesulfonyl chloride reacts with secondary alcohol in the presence of pyridine as a base and forms tosylate derivative (A).
- TsCl is going to make the alcohol as tosylate.
- We know that tosylate groups are good leaving groups in the presence of strong reducing agents.
Step-2 :
- In the second step the formed tosylate is going to react with lithium aluminum hydride and forms an alkane as a product.
- The chemical reaction of reducing tosylate into alkanes is as follows:
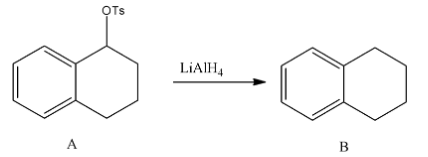
- Means secondary alcohol is going to convert into alkane by a two-step process.
- Therefore the product is alkane.
So, the correct option is B.
Note: In industries this method of preparation of alkanes is most popular because there is no need to use a large number of strong reagents. By using simple reagents like tosyl chloride and lithium aluminum hydride we can prepare alkanes from primary or secondary alcohol. P-toluenesulfonyl chloride is going to prepare from p-toluene sulfonic acid.
Complete step by step solution:
- In the question it is given to find the product B in the given chemical reaction.
- The given chemical reaction contains two steps.
Step-1:

- In the first step TsCl = p-toluenesulfonyl chloride reacts with secondary alcohol in the presence of pyridine as a base and forms tosylate derivative (A).
- TsCl is going to make the alcohol as tosylate.
- We know that tosylate groups are good leaving groups in the presence of strong reducing agents.
Step-2 :
- In the second step the formed tosylate is going to react with lithium aluminum hydride and forms an alkane as a product.
- The chemical reaction of reducing tosylate into alkanes is as follows:

- Means secondary alcohol is going to convert into alkane by a two-step process.
- Therefore the product is alkane.
So, the correct option is B.
Note: In industries this method of preparation of alkanes is most popular because there is no need to use a large number of strong reagents. By using simple reagents like tosyl chloride and lithium aluminum hydride we can prepare alkanes from primary or secondary alcohol. P-toluenesulfonyl chloride is going to prepare from p-toluene sulfonic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




