
Product A will be
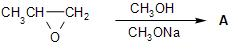
(A)- $C{{H}_{3}}CH(OH)-C{{H}_{2}}(OC{{H}_{3}})$
(B)- $C{{H}_{3}}CH(OC{{H}_{3}})-C{{H}_{2}}(OH)$
(C)- $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OC{{H}_{3}}$
(D)- $C{{H}_{3}}CH(OC{{H}_{3}})C{{H}_{3}}$
Answer
578.1k+ views
Hint: The reaction is a base catalysed nucleophilic substitution reaction due to the presence of the highly reactive epoxide compound, having high ring strain present in it.
Complete step by step answer:
-In the given reaction, the reactant is propylene oxide having a three-membered ring, with the oxygen bonded to two-adjacent carbon atoms. This cyclic ether is called an epoxide, which has a lot of strain and hence highly reactive in nature.
-The reaction of the epoxide with the sodium methoxide base in presence of the methanol solvent causes ring-opening, through the nucleophilic substitution on the electrophilic carbon centers on the epoxide. The reaction mechanism is as follows:
- The base, with $(-OC{{H}_{3}})$ as the nucleophile will attack through ${{S}_{N}}2$, that is from the backside, preferring the least hindered carbon atom. So, the carbon atom with only hydrogen attached to it is favoured.
-This leads to the opening of the strained ring, and the formation of the alkoxide ion, with the negatively charged oxygen atom in it.
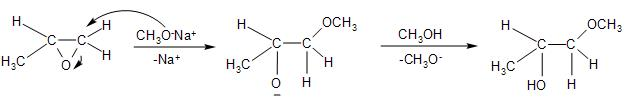
- The methanol present further leads to the protonation of the oxygen atom on the alkoxide ion.
Thus, we get product A to be option (A)- $C{{H}_{3}}CH(OH)-C{{H}_{2}}(OC{{H}_{3}})$, that is, 1-methoxy-2-propanol or the propylene glycol methyl ether.
Note: The ring opening of the epoxide is a regioselective reaction, depending on whether it is a basic or acidic alcohol, involved in the nucleophilic substitution. As we have seen the basic alcohol favours the ${{S}_{N}}2$ mechanism, but the acidic alcohol favours the ${{S}_{N}}1$ mechanism. Also, the methanol solvent is present in equilibrium with the sodium methoxide and does not affect its attack.
Complete step by step answer:
-In the given reaction, the reactant is propylene oxide having a three-membered ring, with the oxygen bonded to two-adjacent carbon atoms. This cyclic ether is called an epoxide, which has a lot of strain and hence highly reactive in nature.
-The reaction of the epoxide with the sodium methoxide base in presence of the methanol solvent causes ring-opening, through the nucleophilic substitution on the electrophilic carbon centers on the epoxide. The reaction mechanism is as follows:
- The base, with $(-OC{{H}_{3}})$ as the nucleophile will attack through ${{S}_{N}}2$, that is from the backside, preferring the least hindered carbon atom. So, the carbon atom with only hydrogen attached to it is favoured.
-This leads to the opening of the strained ring, and the formation of the alkoxide ion, with the negatively charged oxygen atom in it.

- The methanol present further leads to the protonation of the oxygen atom on the alkoxide ion.
Thus, we get product A to be option (A)- $C{{H}_{3}}CH(OH)-C{{H}_{2}}(OC{{H}_{3}})$, that is, 1-methoxy-2-propanol or the propylene glycol methyl ether.
Note: The ring opening of the epoxide is a regioselective reaction, depending on whether it is a basic or acidic alcohol, involved in the nucleophilic substitution. As we have seen the basic alcohol favours the ${{S}_{N}}2$ mechanism, but the acidic alcohol favours the ${{S}_{N}}1$ mechanism. Also, the methanol solvent is present in equilibrium with the sodium methoxide and does not affect its attack.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




