
Primary nitro alkanes are obtained in good yield by oxidizing aldoximes with the help of:
A.) Trifluoroperoxyacetic acid
B.) Acidified potassium permanganate
C.) Concentrated nitric acid
D.) Potassium dichromate and dilute sulphuric acid
Answer
590.7k+ views
Hint: An oxime is a chemical compound belonging to imines.
Oximes have a general formula of \[RCR'=N-OH\].
Where R is an organic side chain and R' is a hydrogen, then it forms an aldoxime,
And if R = R’ = organic, the side chain forms a ketoxime.
The structure of an aldoxime is as follows.
\[RCH=N-OH\]
Complete step by step answer:
Primary nitro alkanes are obtained in good yield by oxidizing aldoximes with the help of trifluoroperoxyacetic acid.
The chemical reaction of aldoximes with trifluoroperoxyacetic acid is as follows.
\[RCH=N-OH+[O]\xrightarrow{trifluoroperoxyacetic\text{ }acid}RCH={{N}^{+}}(-{{O}^{-}})-OHR-C{{H}_{2}}-N{{O}_{2}}\]
Where R = organic side chain
In the above reaction aldoximes react with trifluoroperoxyacetic acid and form an unstable intermediate zwitter ion or compound. The unstable zwitterion or compounds further convert into primary nitroalkanes in good yield.
The structure of Trifluoroperoxyacetic acid is as follows.
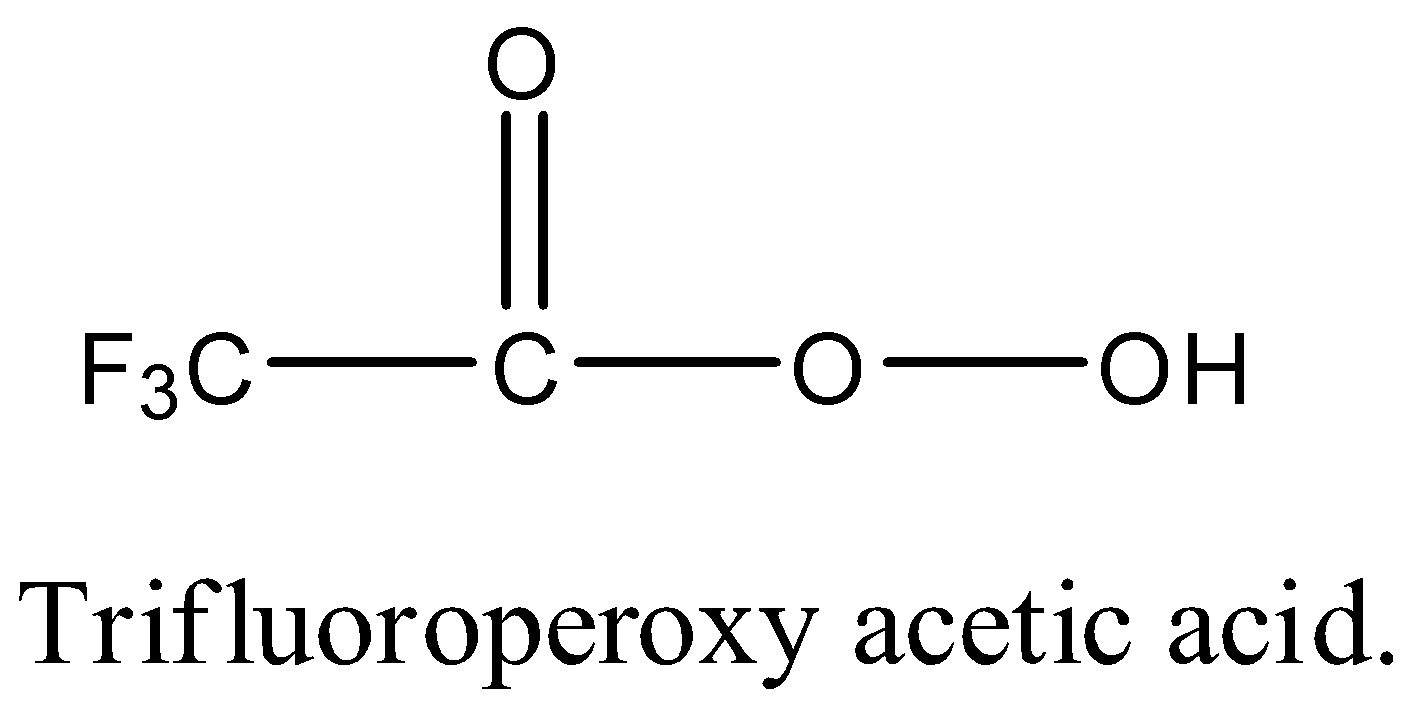
Therefore trifluoroperoxyacetic acid is used to prepare primary nitroalkanes in high yield. This is one of the methods to prepare primary nitroalkanes from aldoximes.
So, the correct answer is “Option A”.
Note: Secondary nitro alkanes are acquired by oxidizing ketoximes by using trifluoroperoxyacetic acid.
\[RCR'=N-OH+[O]\xrightarrow{trifluoroperoxyacetic\text{ }acid}RCR'={{N}^{+}}(-{{O}^{-}})-OHR-CHR'-N{{O}_{2}}\]
Where R, R’ = organic side chains
Trifluoroperoxyacetic acid is one of the strongest oxidizing reagents used in Baeyer–Villiger oxidation reaction.
In Baeyer-Villiger oxidation reactions, ketones are converted into either straight-chain esters or lactones depending on the reactant.
The compound which has both negative and positive charge is called zwitterion.
Oximes have a general formula of \[RCR'=N-OH\].
Where R is an organic side chain and R' is a hydrogen, then it forms an aldoxime,
And if R = R’ = organic, the side chain forms a ketoxime.
The structure of an aldoxime is as follows.
\[RCH=N-OH\]
Complete step by step answer:
Primary nitro alkanes are obtained in good yield by oxidizing aldoximes with the help of trifluoroperoxyacetic acid.
The chemical reaction of aldoximes with trifluoroperoxyacetic acid is as follows.
\[RCH=N-OH+[O]\xrightarrow{trifluoroperoxyacetic\text{ }acid}RCH={{N}^{+}}(-{{O}^{-}})-OHR-C{{H}_{2}}-N{{O}_{2}}\]
Where R = organic side chain
In the above reaction aldoximes react with trifluoroperoxyacetic acid and form an unstable intermediate zwitter ion or compound. The unstable zwitterion or compounds further convert into primary nitroalkanes in good yield.
The structure of Trifluoroperoxyacetic acid is as follows.

Therefore trifluoroperoxyacetic acid is used to prepare primary nitroalkanes in high yield. This is one of the methods to prepare primary nitroalkanes from aldoximes.
So, the correct answer is “Option A”.
Note: Secondary nitro alkanes are acquired by oxidizing ketoximes by using trifluoroperoxyacetic acid.
\[RCR'=N-OH+[O]\xrightarrow{trifluoroperoxyacetic\text{ }acid}RCR'={{N}^{+}}(-{{O}^{-}})-OHR-CHR'-N{{O}_{2}}\]
Where R, R’ = organic side chains
Trifluoroperoxyacetic acid is one of the strongest oxidizing reagents used in Baeyer–Villiger oxidation reaction.
In Baeyer-Villiger oxidation reactions, ketones are converted into either straight-chain esters or lactones depending on the reactant.
The compound which has both negative and positive charge is called zwitterion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




