
Primary and secondary valency of Pt in $\left[ {{\text{Pt(en}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$are:
A. $2,4$
B. $2,6$
C. $6,6$
D. $4,2$
Answer
568.2k+ views
Hint: During the formation of a chemical compound, the number of bonds formed by an atom with another atom is known as valency. The ligands which are directly attached with metals refer to the secondary valency. The ligands that neutralize the complex give the primary valency.
Complete Answer :
Central atoms bind with ligands form a coordination entity. The number of sigma interactions which are made by central metal with ligands is known as coordination number. The ligand approaches the metal to form a complex. Each ligand forms a one sigma bond. The number of ligands that form sigma bonds gives the secondary valency.
The structure of the complex is as follows:
Ethylene is a bidentate ligand. It forms two sigma bonds with central metal.
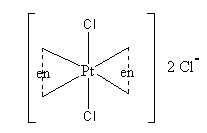
Platinum metal is directly connected with two chloro ligands and two ethylene diamine. Platinum metal forms total form six sigma interactions, so, secondary valency is six.
We will determine the oxidation number of the metal as follows:
The chloro is an anionic ligand and ethylene diamine is a neutral ligand.
So, $\left( {x\, \times 1} \right) + \left( { - 1\, \times 2} \right) + \left( { - 1\, \times 2} \right) = 0$
$x\, = \, + 4$
So, the oxidation number of platinum metal is $ + 4$. Out of which $ + 2$charge is balanced by two chloro ligands which form a sigma bond with the metal (secondary valency). The remaining $ + 2$remains on the complex which is balanced by another two chloro ligands (primary valency).
So, the primary valency of the complex $\left[ {{\text{Pt(en}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$is $2$ and secondary valency is$6$.
Therefore, option (B) $2,6$ is correct.
Note: The ligand can be negatively charged or neutral, rarely positive charged. Dentcity of the ligand is determined as the number of donor atoms of a ligand. The metal which shows interaction with the ligand is known as the central atom. Secondary valency shows the coordination number of the metal whereas primary valency shows the oxidation number of the metal. Primary valency is changeable whereas secondary valency is non-changeable.
Complete Answer :
Central atoms bind with ligands form a coordination entity. The number of sigma interactions which are made by central metal with ligands is known as coordination number. The ligand approaches the metal to form a complex. Each ligand forms a one sigma bond. The number of ligands that form sigma bonds gives the secondary valency.
The structure of the complex is as follows:
Ethylene is a bidentate ligand. It forms two sigma bonds with central metal.

Platinum metal is directly connected with two chloro ligands and two ethylene diamine. Platinum metal forms total form six sigma interactions, so, secondary valency is six.
We will determine the oxidation number of the metal as follows:
The chloro is an anionic ligand and ethylene diamine is a neutral ligand.
So, $\left( {x\, \times 1} \right) + \left( { - 1\, \times 2} \right) + \left( { - 1\, \times 2} \right) = 0$
$x\, = \, + 4$
So, the oxidation number of platinum metal is $ + 4$. Out of which $ + 2$charge is balanced by two chloro ligands which form a sigma bond with the metal (secondary valency). The remaining $ + 2$remains on the complex which is balanced by another two chloro ligands (primary valency).
So, the primary valency of the complex $\left[ {{\text{Pt(en}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]{\text{C}}{{\text{l}}_{\text{2}}}$is $2$ and secondary valency is$6$.
Therefore, option (B) $2,6$ is correct.
Note: The ligand can be negatively charged or neutral, rarely positive charged. Dentcity of the ligand is determined as the number of donor atoms of a ligand. The metal which shows interaction with the ligand is known as the central atom. Secondary valency shows the coordination number of the metal whereas primary valency shows the oxidation number of the metal. Primary valency is changeable whereas secondary valency is non-changeable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




