
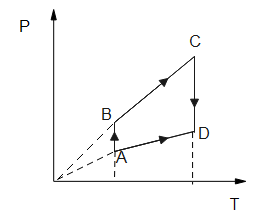
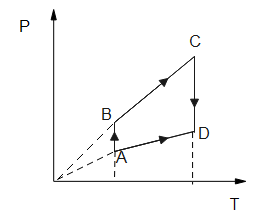
Pressure versus temperature graph for an ideal gas is as shown in the figure.
Corresponding density (ρ) versus volume (v) graph will be
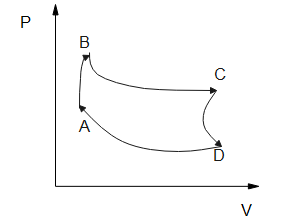
A)
B)
C)
D)





Answer
565.2k+ views
Hint: First we plot the corresponding density v/s pressure graph it makes to draw the corresponding density v/s volume graph. By considering Boyle's law which states that the pressure (P) of a given quantity of gas varies inversely with its volume denoted as V at a constant temperature.
Complete Step by step solution:
First, we have to observe a given graph which is pressure v/s temperature.
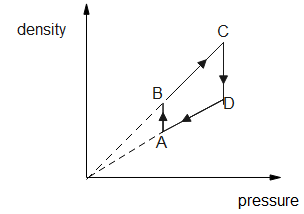
First, we have to plot, Corresponding density versus pressure graph to draw the corresponding density v/s volume graph.
Let’s consider Boyle's law which simply states that the pressure (P) of a given quantity of gas varies inversely with its volume denoted as V at an express constant temperature. We can express it in the mathematical form $P \propto \dfrac{1}{V}$ at T is constant.
So, we can draw the density v/s volume graph as
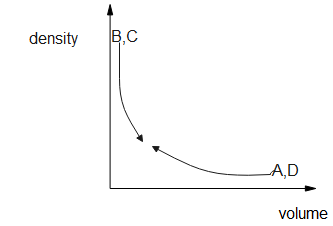
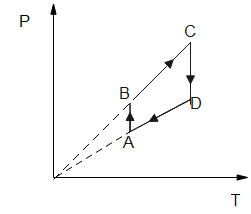
Hence, the option B is the correct answer.
Additional information:
Boyle law state that the volume of a sample of a gas is inversely proportional to its pressure, Charles and Gay-Lussac is demonstrated that the volume of a gas is directly proportional to its temperature (in kelvins) at constant pressure.
Here Boyle's law is used to predict the result of introducing a change in volume and pressure only and to the initial state of a fixed quantity of gas.
Note:
We know that gas can work against excessive ambient pressure by extending or compressing it , work done is often referred to as the amount of pressure-volume or PV when the volume of gas is increased. The ideal gas equation is also known as the equation of the states because this equation uses the variables in it to determine or we can explain the state of the gas that is considered.
Complete Step by step solution:
First, we have to observe a given graph which is pressure v/s temperature.

First, we have to plot, Corresponding density versus pressure graph to draw the corresponding density v/s volume graph.

Let’s consider Boyle's law which simply states that the pressure (P) of a given quantity of gas varies inversely with its volume denoted as V at an express constant temperature. We can express it in the mathematical form $P \propto \dfrac{1}{V}$ at T is constant.
So, we can draw the density v/s volume graph as

Hence, the option B is the correct answer.
Additional information:
Boyle law state that the volume of a sample of a gas is inversely proportional to its pressure, Charles and Gay-Lussac is demonstrated that the volume of a gas is directly proportional to its temperature (in kelvins) at constant pressure.
Here Boyle's law is used to predict the result of introducing a change in volume and pressure only and to the initial state of a fixed quantity of gas.
Note:
We know that gas can work against excessive ambient pressure by extending or compressing it , work done is often referred to as the amount of pressure-volume or PV when the volume of gas is increased. The ideal gas equation is also known as the equation of the states because this equation uses the variables in it to determine or we can explain the state of the gas that is considered.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




