
Prepare carbolic acid from benzene sulphonic acid. Write a chemical equation for the action of neutral ferric chloride on phenol.
Answer
598.5k+ views
Hint: To answer this question first you need to write the structural formula of both the compounds. Then you should do the conversion in steps. You should also know that carbolic acid is known as phenol. Now you can easily get your answer.
Complete step by step solution: Let’s do this conversion step by step -
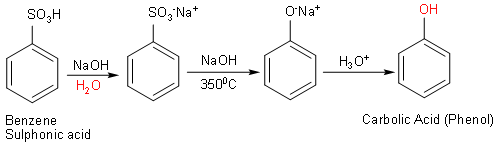
First, we treated benzene sulphonic acid with aqueous NaOH which gives sodium salt of benzene sulphonic acid.
Then, we heated this sodium salt of benzene sulphonic acid and heated with NaOH at $350^{ 0 }$ C to form the sodium salt of phenol (sodium phenoxide).
Finally, the acid hydrolysis of sodium salt gives carbolic acid (phenol).
The action of neutral ferric chloride on phenol gives violet colored complex $[(C_{ 6 }H_{ 5 }O)_{ 6 }Fe]^{ 3- }$.
$6C_{ 6 }H_{ 5 }OH\quad +\quad FeCl_{ 3 }\quad \rightarrow\quad [(C_{ 6 }H_{ 5 }O)_{ 6 }Fe]^{ 3- }+\quad 3HCl$
Therefore, we prepared carbolic acid from benzene sulphonic acid and also wrote a chemical equation for the action of neutral ferric chloride on phenol.
Note: Let’s discuss something related to the nomenclature of the complex compound formed in the second reaction.
$C_{ 6 }H_{ 5 }O^{ - }$ is usually called phenoxide; it is the conjugate base of phenol $6C_{ 6 }H_{ 5 }OH$. Since it’s being used as a ligand and it has a negative charge, change the final -e to -o to get the ligand name phenoxido.
Then we can form the name of $[Fe(C_{ 6 }H_{ 5 }O)_{ 6 }]^{ 3- }$. The central metal is iron, but since the complex is negatively charged, we call it ferrate. In particular, it’s hexaphenoxidoferrate(III) because there are six phenoxido ligands, and also the iron has a +3 oxidation number.
Complete step by step solution: Let’s do this conversion step by step -
First, we treated benzene sulphonic acid with aqueous NaOH which gives sodium salt of benzene sulphonic acid.
Then, we heated this sodium salt of benzene sulphonic acid and heated with NaOH at $350^{ 0 }$ C to form the sodium salt of phenol (sodium phenoxide).
Finally, the acid hydrolysis of sodium salt gives carbolic acid (phenol).

The action of neutral ferric chloride on phenol gives violet colored complex $[(C_{ 6 }H_{ 5 }O)_{ 6 }Fe]^{ 3- }$.
$6C_{ 6 }H_{ 5 }OH\quad +\quad FeCl_{ 3 }\quad \rightarrow\quad [(C_{ 6 }H_{ 5 }O)_{ 6 }Fe]^{ 3- }+\quad 3HCl$
Therefore, we prepared carbolic acid from benzene sulphonic acid and also wrote a chemical equation for the action of neutral ferric chloride on phenol.
Note: Let’s discuss something related to the nomenclature of the complex compound formed in the second reaction.
$C_{ 6 }H_{ 5 }O^{ - }$ is usually called phenoxide; it is the conjugate base of phenol $6C_{ 6 }H_{ 5 }OH$. Since it’s being used as a ligand and it has a negative charge, change the final -e to -o to get the ligand name phenoxido.
Then we can form the name of $[Fe(C_{ 6 }H_{ 5 }O)_{ 6 }]^{ 3- }$. The central metal is iron, but since the complex is negatively charged, we call it ferrate. In particular, it’s hexaphenoxidoferrate(III) because there are six phenoxido ligands, and also the iron has a +3 oxidation number.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




