
Preparation of benzene from phenol is:
Reduction
Oxidation
Addition
Dehydrogenation
Answer
585.6k+ views
Hint: We know that benzene is a cyclic hydrocarbon and has a chemical formula \[{C_6}{H_6}\]. Each carbon atom in benzene is arranged in a six-membered ring and is attached to only one hydrogen atom
Complete step by step answer:
Based molecular orbital theory for benzene structure, benzene ring involves the formation of three delocalized $\pi $ orbitals across all six carbon atoms, while the valence bond theory describes two stable resonance structures for the benzene ring.
Benzene is a volatile and flammable compound. It is highly toxic and smells like gasoline. It is carcinogenic.
We can find benzene in crude oil that is unrefined petroleum and it is also obtained as a natural product by refining oil.
Benzene is immiscible in water and does not form a homogeneous solution with water but in organic solvents, it is soluble.
Benzene is produced through volcanoes and forest fires naturally. There are several laboratories and industrial techniques for the preparation of benzene.
In commercial scale, benzene is mainly obtained from coal tar.
We can prepare Benzene from phenols through their reduction. In this process, phenol vapours are passed over heated zinc dust. Zinc dust reduces them to produce benzene. We can write the chemical reaction as,
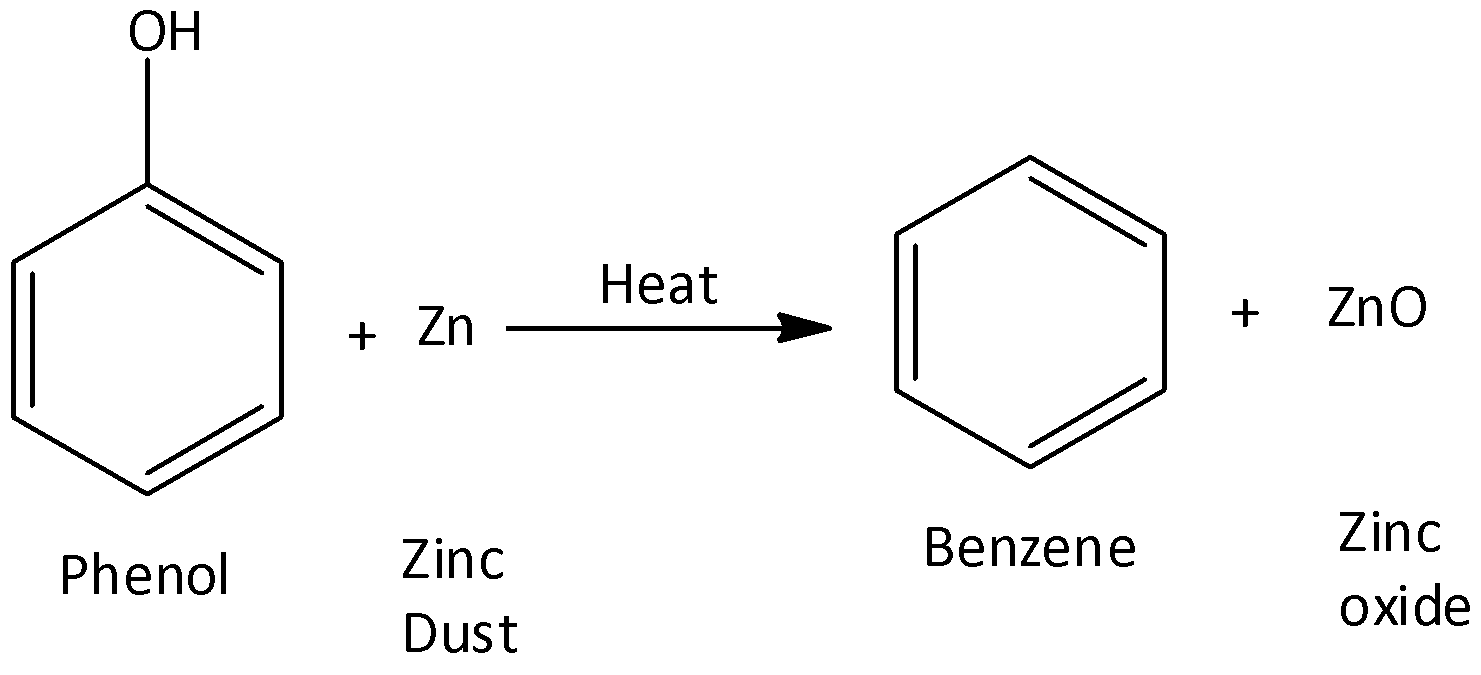
So, the correct answer is Option A .
Note:
We also know the some of the uses of benzene and which are listed below,
Widely used in the production of pesticides, detergents, and resins.
For domestic purposes, we use benzene in glue, adhesive, cleaning products, tobacco etc.
We can also use benzene to prepare aniline and phenol.
It is used in degreasing metals.
Used in the manufacture of chemicals like ethylbenzene, cumene, nitrobenzene, cyclohexane etc.
Complete step by step answer:
Based molecular orbital theory for benzene structure, benzene ring involves the formation of three delocalized $\pi $ orbitals across all six carbon atoms, while the valence bond theory describes two stable resonance structures for the benzene ring.
Benzene is a volatile and flammable compound. It is highly toxic and smells like gasoline. It is carcinogenic.
We can find benzene in crude oil that is unrefined petroleum and it is also obtained as a natural product by refining oil.
Benzene is immiscible in water and does not form a homogeneous solution with water but in organic solvents, it is soluble.
Benzene is produced through volcanoes and forest fires naturally. There are several laboratories and industrial techniques for the preparation of benzene.
In commercial scale, benzene is mainly obtained from coal tar.
We can prepare Benzene from phenols through their reduction. In this process, phenol vapours are passed over heated zinc dust. Zinc dust reduces them to produce benzene. We can write the chemical reaction as,

So, the correct answer is Option A .
Note:
We also know the some of the uses of benzene and which are listed below,
Widely used in the production of pesticides, detergents, and resins.
For domestic purposes, we use benzene in glue, adhesive, cleaning products, tobacco etc.
We can also use benzene to prepare aniline and phenol.
It is used in degreasing metals.
Used in the manufacture of chemicals like ethylbenzene, cumene, nitrobenzene, cyclohexane etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




