
Predict the main product of the following reactions:
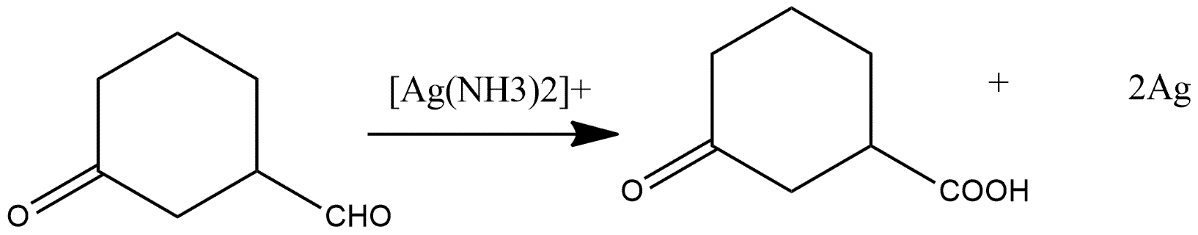
(i)
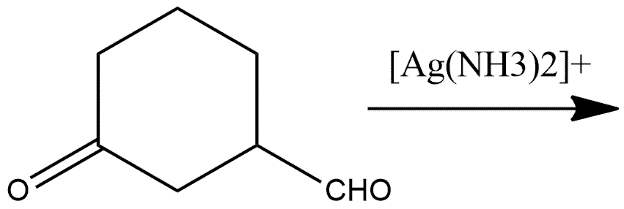
(ii)
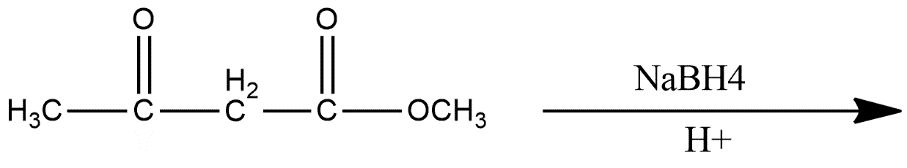
(iii)
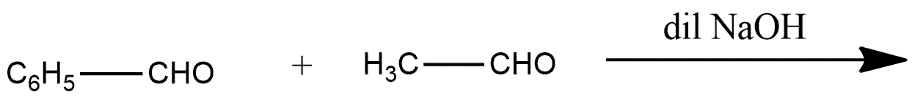

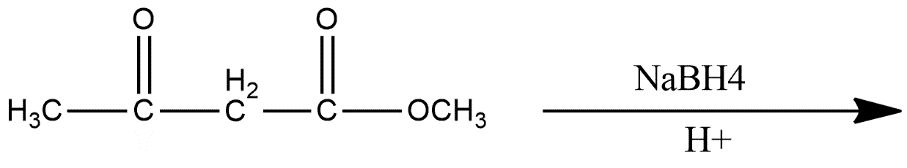
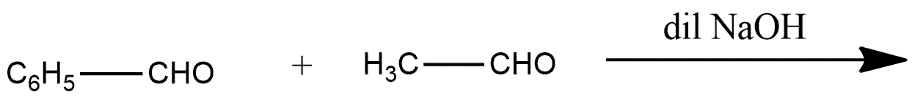
Answer
578.1k+ views
Hint: Tollen’s Reagent is generally a chemical reagent which is used to detect an aldehyde functional group or an aromatic aldehyde functional group or an alpha hydroxy ketone functional group in any substance. Tollen’s reagent is a solution of silver nitrate $AgN{{O}_{3}}$ and ammonia $N{{H}_{3}}$.
Complete Step by step solution:
i) The first reaction given in the reaction contains Tollen’s reagent. In which an aldehydic group get converted into carboxylic acid with the reaction of tollen’s reagent it is generally a reducing agent which converts an aldehydic or ketonic group into acidic one and the reaction shows as follows:
ii) $NaB{{H}_{4}}$ is known by the name Sodium borohydride, sodium tetrahydridoborate and sodium tetrahydroborate. It is generally a white solid in a powdered form. This is a reducing agent and used as a tested reagent for pretreatment for pulping of wood. In the given reaction this generally reduce the ketonic group into alcohol reaction is shown as:
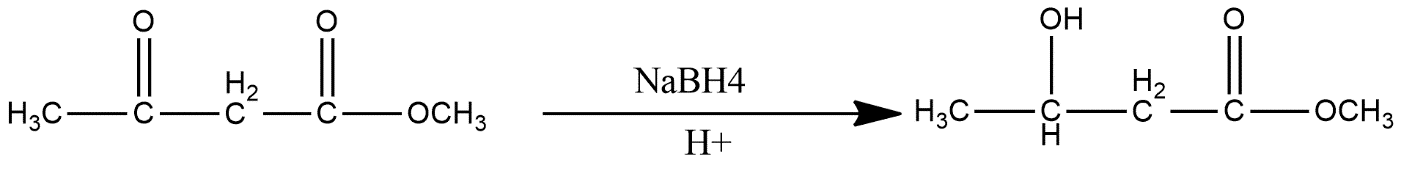
iii) In this reaction two aldehydes joined to form an alkene group in the presence of dilute sodium hydroxide represented by NaOH and the reaction is shown as below:
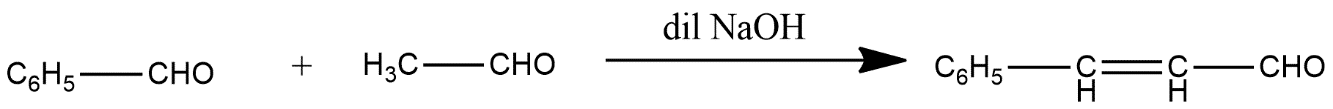
Note: In a redox reaction when a substance loses electrons to other substances and gets oxidized to the higher valency state is known as a reducing agent. If the reducing agent does not pass electrons to other substances in a reaction then we can say that the reduction process is not happening.
Complete Step by step solution:
i) The first reaction given in the reaction contains Tollen’s reagent. In which an aldehydic group get converted into carboxylic acid with the reaction of tollen’s reagent it is generally a reducing agent which converts an aldehydic or ketonic group into acidic one and the reaction shows as follows:

ii) $NaB{{H}_{4}}$ is known by the name Sodium borohydride, sodium tetrahydridoborate and sodium tetrahydroborate. It is generally a white solid in a powdered form. This is a reducing agent and used as a tested reagent for pretreatment for pulping of wood. In the given reaction this generally reduce the ketonic group into alcohol reaction is shown as:
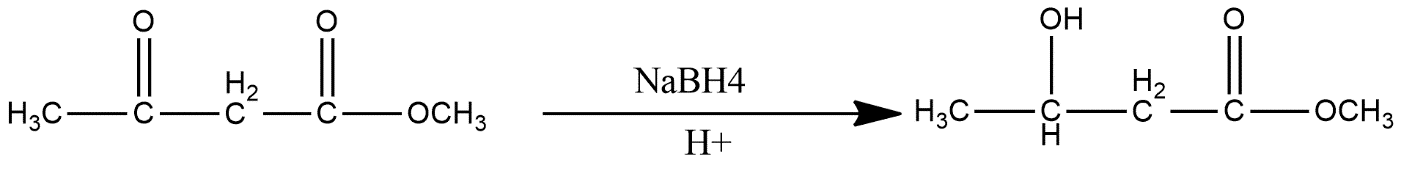
iii) In this reaction two aldehydes joined to form an alkene group in the presence of dilute sodium hydroxide represented by NaOH and the reaction is shown as below:
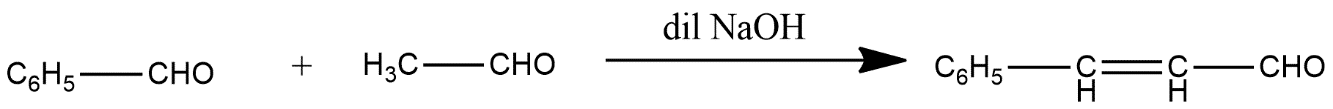
Note: In a redox reaction when a substance loses electrons to other substances and gets oxidized to the higher valency state is known as a reducing agent. If the reducing agent does not pass electrons to other substances in a reaction then we can say that the reduction process is not happening.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




