
Predict the basicity of the final product (having sulphur) obtained when ${\text{S}}{{\text{F}}_4}$ undergoes hydrolysis.
Answer
578.7k+ views
Hint: The basicity is defined as the strength of a base to accept a proton. The basicity of an acid is determined as the number of protons donated by the acid to a base. Here we have to predict the basicity of the product obtained after hydrolysis of ${\text{S}}{{\text{F}}_4}$.
Complete answer:
Hydrolysis of a compound is the dissolution of the compound in water, so the bonds in the compound break, and a new compound forms.
The hydrolysis of sulphur tetrafluoride ${\text{S}}{{\text{F}}_4}$is as follows:
${\text{S}}{{\text{F}}_4} + \,3\,{{\text{H}}_{\text{2}}}{\text{O}}\, \to \,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}\,{\text{ + }}\,4\,{\text{HF}}$
Sulphur tetrafluoride gives acid, sulphurous acid, and hydrogen fluoride as the final products of hydrolysis.
The structure of the final product sulphurous acid (having sulphur) is shown as follows:
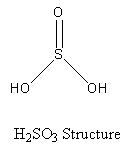
Basicity of an acid is the number of protons donated by the acid to a base.
It can be seen in the structure of the sulphurous acid, that the sulphurous acid has two hydroxyl groups. So, the sulphurous acid can donate two protons to a base, so the basicity of the sulphurous acid is two.
Therefore, the basicity of the final product (sulphurous acid) obtained when ${\text{S}}{{\text{F}}_4}$ undergoes hydrolysis is 2.
Note: The central atom of the main product of hydrolysis has the same oxidation state as the oxidation state of the compound which is hydrolyzed. The acids of sulphur have one sulphur-oxygen double bond and remaining oxygen atoms and hydrogen atoms in hydroxyl form and then the left hydrogen atoms bound directly with sulphur atom. The hydrogen bound in the form of a hydroxyl group can only cause acidity.
Complete answer:
Hydrolysis of a compound is the dissolution of the compound in water, so the bonds in the compound break, and a new compound forms.
The hydrolysis of sulphur tetrafluoride ${\text{S}}{{\text{F}}_4}$is as follows:
${\text{S}}{{\text{F}}_4} + \,3\,{{\text{H}}_{\text{2}}}{\text{O}}\, \to \,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}\,{\text{ + }}\,4\,{\text{HF}}$
Sulphur tetrafluoride gives acid, sulphurous acid, and hydrogen fluoride as the final products of hydrolysis.
The structure of the final product sulphurous acid (having sulphur) is shown as follows:

Basicity of an acid is the number of protons donated by the acid to a base.
It can be seen in the structure of the sulphurous acid, that the sulphurous acid has two hydroxyl groups. So, the sulphurous acid can donate two protons to a base, so the basicity of the sulphurous acid is two.
Therefore, the basicity of the final product (sulphurous acid) obtained when ${\text{S}}{{\text{F}}_4}$ undergoes hydrolysis is 2.
Note: The central atom of the main product of hydrolysis has the same oxidation state as the oxidation state of the compound which is hydrolyzed. The acids of sulphur have one sulphur-oxygen double bond and remaining oxygen atoms and hydrogen atoms in hydroxyl form and then the left hydrogen atoms bound directly with sulphur atom. The hydrogen bound in the form of a hydroxyl group can only cause acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




