
Predict hybridization and shape of $S{F_4}$ molecule.
A. $s{p^3}d$ and see saw
B. $s{p^3}$ and tetrahedral
C. sp and T-shaped
D. $s{p^3}{d^2}$ and octahedral
Answer
559.2k+ views
Hint: In sulphur tetrafluoride molecules the central atom sulphur is bonded with four fluorine atoms where the sulphur contains one pair of electrons. The sulphur atom uses a total of five orbitals.
Complete step by step answer:
In the $S{F_4}$ molecule, one sulphur and four fluorine atoms are present. The total valence electrons present in sulphur tetrafluoride is 34 where the 6 valence electrons are of sulphur and each four fluorine atom will have 7 valence electrons. When the $S{F_4}$ molecule is formed the sulphur bonds with four fluorine atoms by using 8 valence electrons. The four fluorine atoms will have 3 lone pairs of electrons present in their octet. Two electrons are present as lone pairs in sulphur. During the bonding, four single bonds are formed in sulphur with one lone pair. Thus the number of regions of electron density is 5. The middle sulphur atom contains 5 valence atomic orbital hybridized to form $s{p^3}d$ hybrid orbital. The sulphur uses its five orbitals one 3s-orbital, three 3p-orbital and one 3d-orbital.
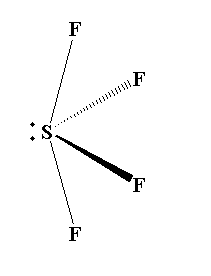
The molecular geometry of $S{F_4}$ is see-saw with one pair of valence electrons. The equatorial fluorine atom has 102 degree bond angle instead of actual 120 degree bond angle. The axial fluorine atom has 173 degree bond angle instead of actual 180 degree bond angle.
The structure of $S{F_4}$ is shown below.
Therefore, the correct option is A.
Note:
The nature of the sulphur tetrafluoride molecule is polar. The shape of the atoms is trigonal bipyramidal. The steric number also helps to determine the number of hybrid orbitals used by the atoms.
Complete step by step answer:
In the $S{F_4}$ molecule, one sulphur and four fluorine atoms are present. The total valence electrons present in sulphur tetrafluoride is 34 where the 6 valence electrons are of sulphur and each four fluorine atom will have 7 valence electrons. When the $S{F_4}$ molecule is formed the sulphur bonds with four fluorine atoms by using 8 valence electrons. The four fluorine atoms will have 3 lone pairs of electrons present in their octet. Two electrons are present as lone pairs in sulphur. During the bonding, four single bonds are formed in sulphur with one lone pair. Thus the number of regions of electron density is 5. The middle sulphur atom contains 5 valence atomic orbital hybridized to form $s{p^3}d$ hybrid orbital. The sulphur uses its five orbitals one 3s-orbital, three 3p-orbital and one 3d-orbital.
The molecular geometry of $S{F_4}$ is see-saw with one pair of valence electrons. The equatorial fluorine atom has 102 degree bond angle instead of actual 120 degree bond angle. The axial fluorine atom has 173 degree bond angle instead of actual 180 degree bond angle.
The structure of $S{F_4}$ is shown below.

Therefore, the correct option is A.
Note:
The nature of the sulphur tetrafluoride molecule is polar. The shape of the atoms is trigonal bipyramidal. The steric number also helps to determine the number of hybrid orbitals used by the atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




