
Potassium dichromate in alkaline solution with \[30\% \] \[{H_2}{O_2}\] produces \[{K_3}Cr{O_8}\]. How many peroxide linkages are found in the structure of \[{K_3}Cr{O_8}\]?
Answer
516k+ views
Hint: To solve this question, first write the complete balanced equation for the given reaction. Here, the reaction is a redox reaction. Now, to find the number of linkages, use the formula for calculating the number of peroxide linkages. Before that, we will need the oxidation number of elements as well to be used in the formula.
Complete answer:
When potassium dichromate reacts with hydrogen peroxide, the following redox reaction is carried out:
\[{K_2}C{r_2}{O_7} + 7{H_2}{O_2} + 4KOH(alk) \to 2{K_3}Cr{O_8} + 9{H_2}O\]
In the reaction, Hydrogen peroxide acts as an oxidizing agent and gets reduced. Its oxidation number changes to \[ - 2\] from \[ - 1\].
The number of peroxide linkages formed here is \[4\] and can be verified by drawing the structure of \[{K_3}Cr{O_8}\].
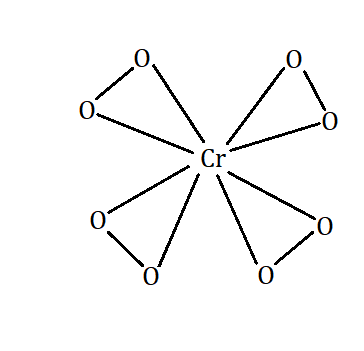
The number of peroxide linkages in a compound can be calculated by using the formula \[ = \] (Theoretical oxidation number) \[ - \] (Maximum oxidation number) \[/2\].
The theoretical oxidation state of \[C{r^ - }\] in \[{K_3}Cr{O_8}\] will be \[ = 16 - 3 = 13\]
The maximum number of linkages in the structure \[{[Cr{O_8}]^{3 - }}\] are \[ = 5\]
The number of peroxide linkages according to the formula can be calculated as \[ = \dfrac{{(13 - 5)}}{2} = 4\]
Hence, the number of peroxide linkage is \[4\].
Note:
Whenever hydrogen peroxide is added to dichromate or any other \[Cr(VI)\] compound, then a blue colour is obtained. This blue colour is due to the formation of \[CrO({O_2})\]. However, the blue colour fades away and the compound decomposes readily into \[C{r^{3 + }}\] and \[{O_2}(g)\] in aqueous solution. The peroxo compound can be extracted into oxygenated organic solvent like diethyl ether, ethyl acetate or \[1 - \]pentanol etc. where it remains stable.
Complete answer:
When potassium dichromate reacts with hydrogen peroxide, the following redox reaction is carried out:
\[{K_2}C{r_2}{O_7} + 7{H_2}{O_2} + 4KOH(alk) \to 2{K_3}Cr{O_8} + 9{H_2}O\]
In the reaction, Hydrogen peroxide acts as an oxidizing agent and gets reduced. Its oxidation number changes to \[ - 2\] from \[ - 1\].
The number of peroxide linkages formed here is \[4\] and can be verified by drawing the structure of \[{K_3}Cr{O_8}\].

The number of peroxide linkages in a compound can be calculated by using the formula \[ = \] (Theoretical oxidation number) \[ - \] (Maximum oxidation number) \[/2\].
The theoretical oxidation state of \[C{r^ - }\] in \[{K_3}Cr{O_8}\] will be \[ = 16 - 3 = 13\]
The maximum number of linkages in the structure \[{[Cr{O_8}]^{3 - }}\] are \[ = 5\]
The number of peroxide linkages according to the formula can be calculated as \[ = \dfrac{{(13 - 5)}}{2} = 4\]
Hence, the number of peroxide linkage is \[4\].
Note:
Whenever hydrogen peroxide is added to dichromate or any other \[Cr(VI)\] compound, then a blue colour is obtained. This blue colour is due to the formation of \[CrO({O_2})\]. However, the blue colour fades away and the compound decomposes readily into \[C{r^{3 + }}\] and \[{O_2}(g)\] in aqueous solution. The peroxo compound can be extracted into oxygenated organic solvent like diethyl ether, ethyl acetate or \[1 - \]pentanol etc. where it remains stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




