
Polymer of $ 2 - methyl - 1,3 - butadiene(isoprene) $ would give which product on (reductive) ozonolysis?
$ A)C{H_3} - CH\left( {CHO} \right) - CO - C{H_3} $
$ B)OHC - C{H_2} - C{H_2} - CO - C{H_3} $
$ C)OHC - C{H_2} - C{H_2} - C{H_2} - CHO $
$ D)C{H_3} - CO - C{H_2} - CO - C{H_3} $
Answer
506.1k+ views
Hint :Isoprene is the common name of $ 2 - methyl - 1,3 - butadiene $ , the polymer of isoprene undergoes reductive ozonolysis the double bonds are reduced to produce carbonyl compounds i.e.., aldehydes and ketones. Thus, double bonds are reduced to aldehydes and ketones.
Complete Step By Step Answer:
Given molecule is isoprene. The chemical name of isoprene is $ 2 - methyl - 1,3 - butadiene $ . It has two double bonds and one methyl substituent at $ {2^{nd}} $ carbon.
The polymer of isoprene is natural rubber. The structure of natural rubber will be as follows:
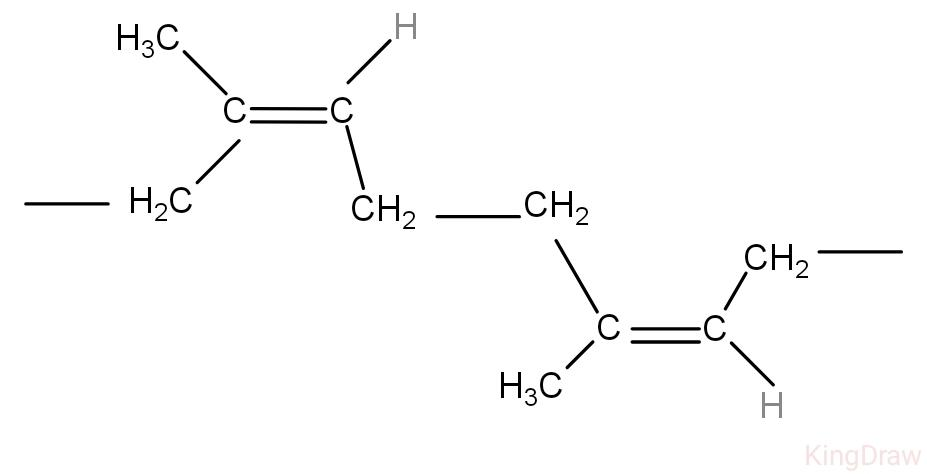
The above molecule is the polymer of isoprene, it has double bonds at two positions. Ozonolysis is the addition of ozone molecules to unsaturated compounds like alkenes and alkynes that form carbonyl compounds.
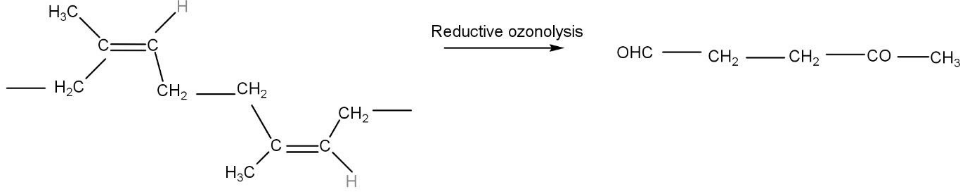
The cleavage of C-C bond also takes place in some conditions. Ozonolysis can also be reductive which means that after the reaction with ozone the unsaturated compounds forms ozonide, ozonide is a complex in which the three atoms of oxygen form a complex with the unsaturated compounds. further on treating metal like Zinc forms carbonyl compounds. In this molecule of natural rubber, the double bonds get reduced to carbonyl compounds, one gets reduced to aldehydes and the other gets reduced to ketone. The product formed is $ 4 - oxapen\tan al $ .
Note :
The polymer of isoprene is different from the isoprene, the double bonded carbon can be converted into the carbonyl compound the aldehyde and ketone are different and one should not confuse it with the formation of ketone and aldehyde.
Complete Step By Step Answer:
Given molecule is isoprene. The chemical name of isoprene is $ 2 - methyl - 1,3 - butadiene $ . It has two double bonds and one methyl substituent at $ {2^{nd}} $ carbon.
The polymer of isoprene is natural rubber. The structure of natural rubber will be as follows:

The above molecule is the polymer of isoprene, it has double bonds at two positions. Ozonolysis is the addition of ozone molecules to unsaturated compounds like alkenes and alkynes that form carbonyl compounds.
The cleavage of C-C bond also takes place in some conditions. Ozonolysis can also be reductive which means that after the reaction with ozone the unsaturated compounds forms ozonide, ozonide is a complex in which the three atoms of oxygen form a complex with the unsaturated compounds. further on treating metal like Zinc forms carbonyl compounds. In this molecule of natural rubber, the double bonds get reduced to carbonyl compounds, one gets reduced to aldehydes and the other gets reduced to ketone. The product formed is $ 4 - oxapen\tan al $ .

Note :
The polymer of isoprene is different from the isoprene, the double bonded carbon can be converted into the carbonyl compound the aldehyde and ketone are different and one should not confuse it with the formation of ketone and aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




