
Phenyl cyanide on reduction with $Na/{C_2}{H_5}OH$ yields:
A.${C_6}{H_5}C{H_2}N{H_2}$
B.${C_6}{H_5}NHC{H_3}$
C.
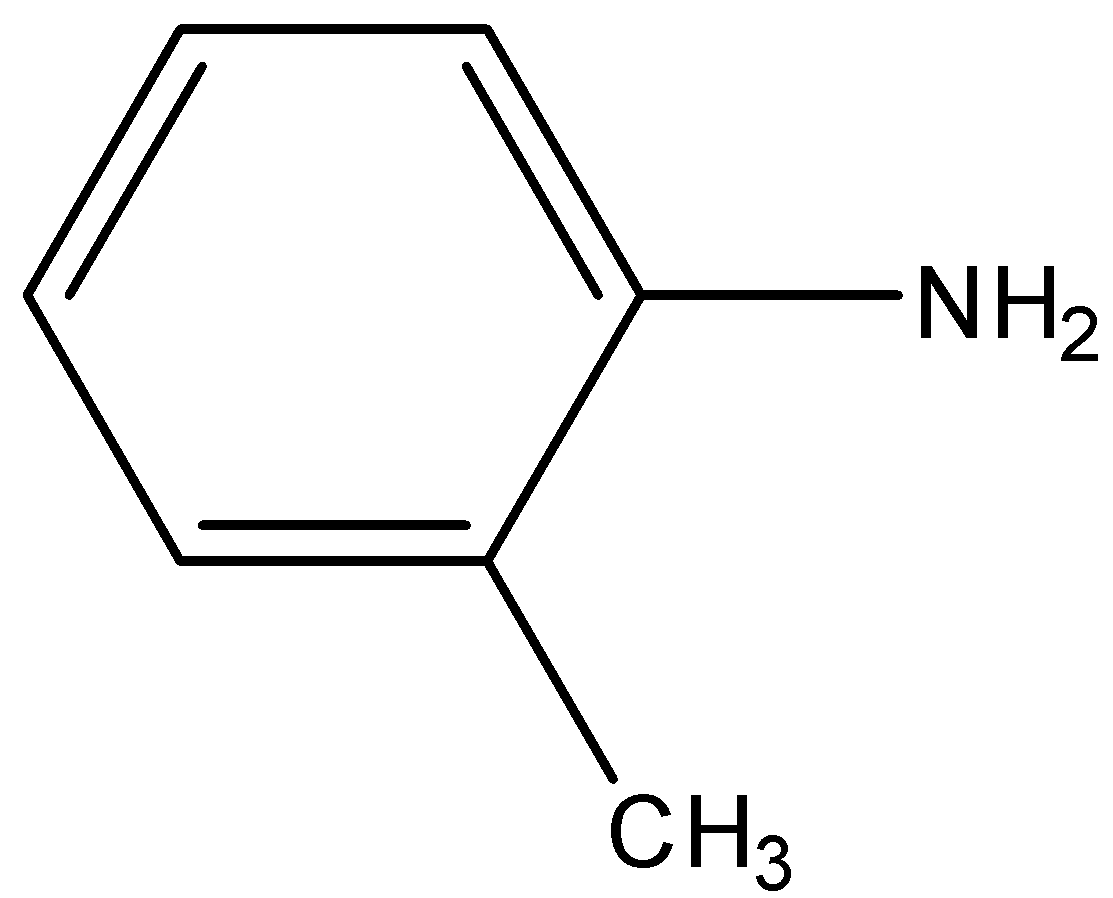
D.${C_6}{H_5}N{H_2}$

Answer
548.1k+ views
Hint: When phenyl cyanide ${C_6}{H_5}CN$ is reacted with sodium ethanol $Na/{C_2}{H_5}OH$ a simple reduction reaction occurs in which cyanide $\left( { - CN} \right)$ is reduced. So, we should know about the basic reduction reaction of cyanides.
Complete step by step answer:
First, we will understand the basics of a reaction. The given question says that phenyl cyanide which is also known as benzonitrile cyanides are used to be termed as nitriles undergo a reduction in the presence of sodium ethanol $Na/{C_2}{H_5}OH$ which acts as a reducing agent in the reaction. So, we can represent the following reaction using proper symbols and representations as given below.
\[{C_6}{H_5}CN\xrightarrow{{Na/{C_2}{H_5}OH}}{C_6}{H_5}C{H_2}N{H_2}\]
From the above reduction reaction, we can observe that phenyl cyanide also known as benzonitrile is a reactant and is represented as ${C_6}{H_5}CN$. Now when phenyl cyanide undergoes a reduction in the presence of sodium ethanol which is represented as $Na/{C_2}{H_5}OH$. Due to the presence of sodium ethanol, the cyanide attached with the benzene ring is reduced to a primary aromatic amine. The final product of the reduction reaction is benzylamine which is a primary aromatic amine and the chemical formula is ${C_6}{H_5}C{H_2}N{H_2}$. So, we can conclude that Phenyl cyanide on reduction with $Na/{C_2}{H_5}OH$ yields benzylamine ${C_6}{H_5}C{H_2}N{H_2}$.
Therefore, the correct option is (A).
Additional information:
Here we will discuss some basic preparation of amines. One of the most basic preparations of primary aromatic amine is the reaction of ammonia with an alkyl halide. The primary amine can also be obtained using Gabriel phthalimide synthesis.
Note:
Phenyl cyanide or benzonitrile is a colorless liquid with an almond-like odor. Generally, it is used as the specialty solvent and for the production of other chemicals. In $Na/{C_2}{H_5}OH$ sodium, the metal reacts easily to give off bubbles of hydrogen gas.
Complete step by step answer:
First, we will understand the basics of a reaction. The given question says that phenyl cyanide which is also known as benzonitrile cyanides are used to be termed as nitriles undergo a reduction in the presence of sodium ethanol $Na/{C_2}{H_5}OH$ which acts as a reducing agent in the reaction. So, we can represent the following reaction using proper symbols and representations as given below.
\[{C_6}{H_5}CN\xrightarrow{{Na/{C_2}{H_5}OH}}{C_6}{H_5}C{H_2}N{H_2}\]
From the above reduction reaction, we can observe that phenyl cyanide also known as benzonitrile is a reactant and is represented as ${C_6}{H_5}CN$. Now when phenyl cyanide undergoes a reduction in the presence of sodium ethanol which is represented as $Na/{C_2}{H_5}OH$. Due to the presence of sodium ethanol, the cyanide attached with the benzene ring is reduced to a primary aromatic amine. The final product of the reduction reaction is benzylamine which is a primary aromatic amine and the chemical formula is ${C_6}{H_5}C{H_2}N{H_2}$. So, we can conclude that Phenyl cyanide on reduction with $Na/{C_2}{H_5}OH$ yields benzylamine ${C_6}{H_5}C{H_2}N{H_2}$.
Therefore, the correct option is (A).
Additional information:
Here we will discuss some basic preparation of amines. One of the most basic preparations of primary aromatic amine is the reaction of ammonia with an alkyl halide. The primary amine can also be obtained using Gabriel phthalimide synthesis.
Note:
Phenyl cyanide or benzonitrile is a colorless liquid with an almond-like odor. Generally, it is used as the specialty solvent and for the production of other chemicals. In $Na/{C_2}{H_5}OH$ sodium, the metal reacts easily to give off bubbles of hydrogen gas.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




