
Phenols are prepared from benzene in the presence of:
A. Alum
B. Oleum
C. Vinegar
D. All of these
Answer
535.5k+ views
Hint: We know that the phenol is the organic compound which is bonded to the hydroxyl group which contains the benzene ring with it. Phenol is treated with diazonium salt, cumene and haloarenes. It is considered as carbolic acid too. They are considered as weak acids which have the ability to form phenoxide ions by removing one strong hydrogen ion from the hydroxyl group .
Complete step by step solution:
In earlier times phenol was formed from coal tar. But now as science and technology have been developed new methods have arisen for the preparation of phenol in the laboratory. Oeum is considered as a fuming sulphuric acid which is referred to as the solutions of various compositions of sulphur trioxide in the sulphuric acid. It is used in the conversion of benzene to phenol by reacting benzene with oleum to form benzene sulphonic acid. This benzene sulphonic acid is then further reacted with sodium hydroxide and then the process of acidification occurs to obtain phenol. The detailed reaction for the above explaination is given below:
Oleum is reacted with benzene to form benzene sulphonic acid
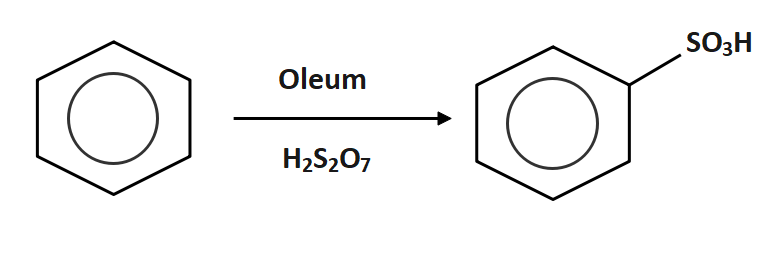
Now the benzene sulphonic acid is reacted with sodium hydroxide
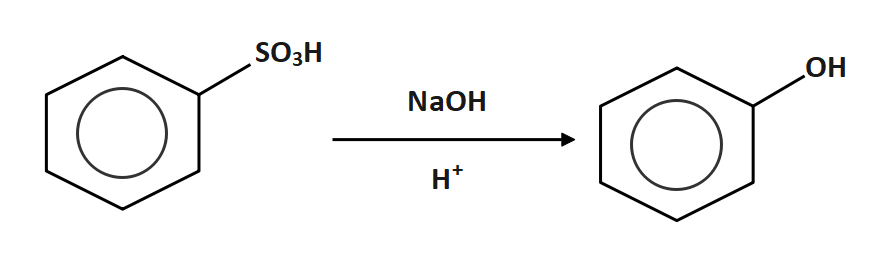
So, the correct answer is Option B
Note: Note that the phenols are used as intermediate for chemical synthesis and in household products also. Such phenol is used as a disinfectant in low concentration as mouthwash cleaners also. The first antiseptic surgery was phenol. It is a natural chemical used in air fresheners, deodorants, acne remedies and in washing.
Complete step by step solution:
In earlier times phenol was formed from coal tar. But now as science and technology have been developed new methods have arisen for the preparation of phenol in the laboratory. Oeum is considered as a fuming sulphuric acid which is referred to as the solutions of various compositions of sulphur trioxide in the sulphuric acid. It is used in the conversion of benzene to phenol by reacting benzene with oleum to form benzene sulphonic acid. This benzene sulphonic acid is then further reacted with sodium hydroxide and then the process of acidification occurs to obtain phenol. The detailed reaction for the above explaination is given below:
Oleum is reacted with benzene to form benzene sulphonic acid

Now the benzene sulphonic acid is reacted with sodium hydroxide

So, the correct answer is Option B
Note: Note that the phenols are used as intermediate for chemical synthesis and in household products also. Such phenol is used as a disinfectant in low concentration as mouthwash cleaners also. The first antiseptic surgery was phenol. It is a natural chemical used in air fresheners, deodorants, acne remedies and in washing.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




