
\[{\text{Phenol }}\xrightarrow[{{\text{oxid}}}]{{{\text{Aerial}}}}{\text{Coloured product}}\] This is due to the formation of :
E.
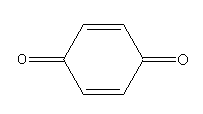
F.
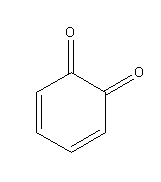
G.
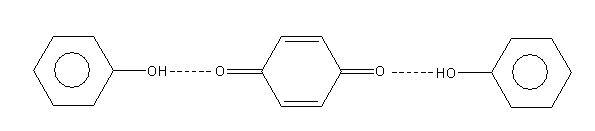
H. All



Answer
568.5k+ views
Hint:The reaction given to us is aerial oxidation of phenol. Oxidation of phenol in presence of air gives the colour product. The oxygen in the air reacts with phenol and gives quinone products.
Complete step by step answer:On exposure to air phenol slowly oxidises and turns to the p-benzoquinone product. The colour of the p-benzoquinone product is pink.
The oxidation reaction of phenol is as follows:
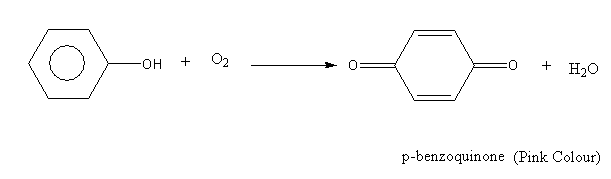
Here, phenol is aromatic while the p-benzoquinone is non-aromatic.
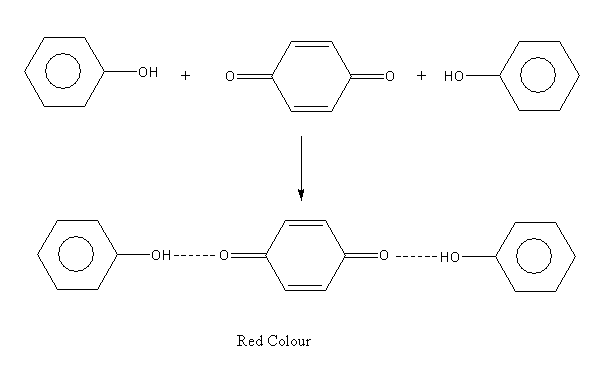
A p-benzoquinone form again reacts with a second molecule of phenol and gives the polymerized product of diphenoquinone which is red colour.
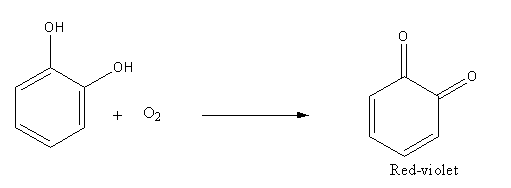
Oxidation of dihydroxybenzene (catechol) in presence of air produce 1,2-benzoquinone:
so (B) is the incorrect option.Thus, option (A) and (C) are the correct answer.
Additional Information:
Oxidation of phenol in the air gives colour compounds. When we store phenol in the air it turns to pink due to the formation of p-benzoquinone.
Catechol when stored in the air turns to red-violet due to the formation of 1, 2-benzoquinone.
Note: Oxidation is the addition of oxygen atoms. The phenol hydroxyl group is an electron-donating group that influences the nucleophilic character of phenol. Quinone's product is non-aromatic. In quinines carbonyl double bond is in conjugation with a double bond in the ring.
Complete step by step answer:On exposure to air phenol slowly oxidises and turns to the p-benzoquinone product. The colour of the p-benzoquinone product is pink.
The oxidation reaction of phenol is as follows:

Here, phenol is aromatic while the p-benzoquinone is non-aromatic.
A p-benzoquinone form again reacts with a second molecule of phenol and gives the polymerized product of diphenoquinone which is red colour.

Oxidation of dihydroxybenzene (catechol) in presence of air produce 1,2-benzoquinone:

so (B) is the incorrect option.Thus, option (A) and (C) are the correct answer.
Additional Information:
Oxidation of phenol in the air gives colour compounds. When we store phenol in the air it turns to pink due to the formation of p-benzoquinone.
Catechol when stored in the air turns to red-violet due to the formation of 1, 2-benzoquinone.
Note: Oxidation is the addition of oxygen atoms. The phenol hydroxyl group is an electron-donating group that influences the nucleophilic character of phenol. Quinone's product is non-aromatic. In quinines carbonyl double bond is in conjugation with a double bond in the ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




