
$PhCHO+{{(C{{H}_{3}}CO)}_{2}}O\xrightarrow{C{{H}_{3}}COONa}A\xrightarrow{HBr}B$
The product B is:
$PhCH=CHC{{H}_{2}}Br$
$PhCHBrC{{H}_{2}}COOH$
$PhC{{H}_{2}}CHBrCOOH$
$PhCH=CHCOBr$
Answer
585.3k+ views
Hint: Benzaldehyde and acetic anhydride in the above reaction condense in the presence of sodium acetate which leads to the formation of product “A” i.e. cinnamic acid. This reaction is known as the Perkin condensation reaction.
Complete step by step solution:
The Perkin condensation reaction is an organic reaction which helps in the manufacturing of cinnamic acid. Perkin condensation reaction form an $\alpha ,\beta -$unsaturated aromatic acid in the presence of an alkali salt of the present acid by the aldol condensation of an aromatic aldehyde and an acid anhydride.
The basic reaction is aromatic anhydride + aliphatic acid anhydride + the alkali salt of the acid and after that the formation of a product which is the cinnamic acid derivative.
The general mechanism of Perkin condensation is shown below-
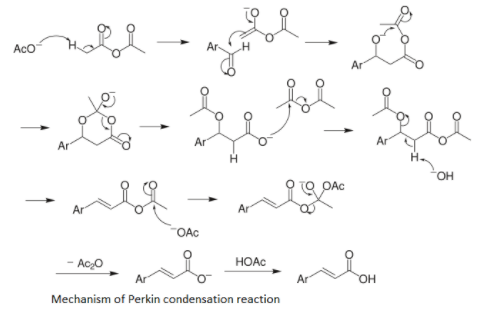
Hence the product A formed is $PhCH=CHCOOH$. The second step is the reaction of $PhCH=CHCOOH$ with HBr. Hydrogen bromide adds to the alkene and it produces an alkyl halide. The pi bond of the alkene acts as a weak nucleophile and HBr has an electrophilic proton which reacts with the weak nucleophile of alkene. If the alkene is unsymmetrical then the one which forms the more stable carbocation will be favored. There are two steps in which the addition of HBr takes place first is the formation of carbocation and the second one is the attack of electrophilic protons of HBr.
$PhCH=CHCOOH+HBr\to PhCHBrC{{H}_{2}}COOH$
Hence the correct answer is option (B)
Note: During the addition of HBr, If the alkene is unsymmetrical then the one which forms the more stable carbocation will be favored. Order of stability of carbocation is tertiary then secondary than primary due to hyper conjugation.
Complete step by step solution:
The Perkin condensation reaction is an organic reaction which helps in the manufacturing of cinnamic acid. Perkin condensation reaction form an $\alpha ,\beta -$unsaturated aromatic acid in the presence of an alkali salt of the present acid by the aldol condensation of an aromatic aldehyde and an acid anhydride.
The basic reaction is aromatic anhydride + aliphatic acid anhydride + the alkali salt of the acid and after that the formation of a product which is the cinnamic acid derivative.
The general mechanism of Perkin condensation is shown below-

Hence the product A formed is $PhCH=CHCOOH$. The second step is the reaction of $PhCH=CHCOOH$ with HBr. Hydrogen bromide adds to the alkene and it produces an alkyl halide. The pi bond of the alkene acts as a weak nucleophile and HBr has an electrophilic proton which reacts with the weak nucleophile of alkene. If the alkene is unsymmetrical then the one which forms the more stable carbocation will be favored. There are two steps in which the addition of HBr takes place first is the formation of carbocation and the second one is the attack of electrophilic protons of HBr.
$PhCH=CHCOOH+HBr\to PhCHBrC{{H}_{2}}COOH$
Hence the correct answer is option (B)
Note: During the addition of HBr, If the alkene is unsymmetrical then the one which forms the more stable carbocation will be favored. Order of stability of carbocation is tertiary then secondary than primary due to hyper conjugation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




