
Percentage weight of hydrogen in urea is:
A. 6.67%
B. 10%
C. 4%
D. 12.33%
Answer
600.6k+ views
Hint: Solve this question by calculating the molecular weight of the compound using weights of the element. Then, divide the weight of the desired element by the molar mass of the compound to find weight of the compound in the element. Multiply it by 100 to find the percentage.
Complete step by step answer:
Urea, also known as carbamide, is a compound with molecular formula \[CO{{(N{{H}_{2}})}_{2}}\]. It is a highly soluble organic compound formed in the liver from ammonia produced as a result of deamination of amino acids. It is a major organic component of human urine and is the waste product of many living organisms.
The structural formula of urea is –
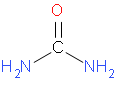
Let us start by calculating molar mass of urea.
= molar mass of carbon + molar mass of oxygen + (2 x molar mass of nitrogen) + (4 x molar mass of hydrogen)
= 12 g + 16 g + (2 x 14) g + (4 x 1) g
= 60 g.
% weight of element = \[\dfrac{\text{mass of element}}{\text{molar mass of compound}}\]x 100 %
% weight of hydrogen =\[\dfrac{\text{4}}{\text{60}}\] x 100 %
% weight of hydrogen = 6.67 %
Therefore, the answer is – option (a) – Percentage weight of hydrogen in urea is 6.67%.
Note: Percentage weights of other elements in urea are as follows –
Carbon
% weight = \[\dfrac{12}{\text{60}}\text{ }\]x 100 % = 19.99 %
Nitrogen
% weight = \[\dfrac{14}{\text{60}}\] x 100 % = 46.64 %
Oxygen
% weight = \[\dfrac{16}{\text{60}}\] x 100 % = 26.64 %
Complete step by step answer:
Urea, also known as carbamide, is a compound with molecular formula \[CO{{(N{{H}_{2}})}_{2}}\]. It is a highly soluble organic compound formed in the liver from ammonia produced as a result of deamination of amino acids. It is a major organic component of human urine and is the waste product of many living organisms.
The structural formula of urea is –

Let us start by calculating molar mass of urea.
= molar mass of carbon + molar mass of oxygen + (2 x molar mass of nitrogen) + (4 x molar mass of hydrogen)
= 12 g + 16 g + (2 x 14) g + (4 x 1) g
= 60 g.
% weight of element = \[\dfrac{\text{mass of element}}{\text{molar mass of compound}}\]x 100 %
% weight of hydrogen =\[\dfrac{\text{4}}{\text{60}}\] x 100 %
% weight of hydrogen = 6.67 %
Therefore, the answer is – option (a) – Percentage weight of hydrogen in urea is 6.67%.
Note: Percentage weights of other elements in urea are as follows –
Carbon
% weight = \[\dfrac{12}{\text{60}}\text{ }\]x 100 % = 19.99 %
Nitrogen
% weight = \[\dfrac{14}{\text{60}}\] x 100 % = 46.64 %
Oxygen
% weight = \[\dfrac{16}{\text{60}}\] x 100 % = 26.64 %
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




